- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Maize Seedlings Colonization with Serendipita indica and Its Colonization Efficiency Analysis
Published: Vol 13, Iss 20, Oct 20, 2023 DOI: 10.21769/BioProtoc.4855 Views: 2263
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Quantitative Estimation of Auxin, Siderophore, and Hydrogen Cyanide Production in Halo and Drought-Tolerant Bacterial Isolates for Cucumber Growth
Zeinab Fotoohiyan and Ali Salehi Sardoei
Oct 5, 2025 1340 Views
Reproducible Emu-Based Workflow for High-Fidelity Soil and Plant Microbiome Profiling on HPC Clusters
Henrique M. Dias [...] Christopher Graham
Jan 20, 2026 374 Views
Abstract
Maize is one of the most important crops in the world, and ensuring its successful growth and productivity is crucial for global food security. One way to enhance maize growth and productivity is by improving the colonization of its roots by beneficial microorganisms. In this regard, Serendipita indica, a plant growth–promoting fungus, has gained attention for its ability to enhance plant growth and productivity, especially in cereal crops and medicinal plants. Previous studies have shown that S. indica can colonize various plant species, including maize, but the efficiency of the colonization process in maize seedlings has not been extensively characterized. This protocol outlines a method for efficient colonization of maize seedlings with the beneficial fungus S. indica. The protocol includes the preparation of stock solutions, maintenance and growth of S. indica, surface sterilization and germination of seeds, preparation of S. indica chlamydospores, and colonization of maize plants with S. indica. The advantages of this protocol include the use of surface sterilization techniques that minimize contamination, the production of a large number of viable chlamydospores, and efficient colonization of maize seedlings with S. indica. This protocol may be useful for researchers studying the role of S. indica in promoting plant growth and combating biotic and abiotic stress. Additionally, this protocol may be used in the development of biofertilizers using S. indica as a means of increasing crop yields and reducing dependence on synthetic fertilizers. Overall, this protocol offers a reliable and efficient method for colonizing maize seedlings with S. indica and may have potential applications in the agricultural industry. This study also provides a valuable tool for researchers interested in studying plant–microbe interactions in maize and highlights the potential of S. indica as a biocontrol agent to enhance maize productivity under adverse conditions.
Key features
• This protocol builds upon the method developed by Narayan et al. (2022), and its application optimized for the root endophytic symbiotic fungus S. indica.
• This protocol also allows for histochemical analysis to visualize the colonized fungal spores in the root cells of host plant species.
• This protocol helps in mathematical calculation of the percent colonization or efficiency of colonization.
• This protocol utilizes readily available laboratory equipment, including a light microscope, autoclave, and laminar flow hood, ensuring ease of reproducibility in other research laboratories.
Graphical overview
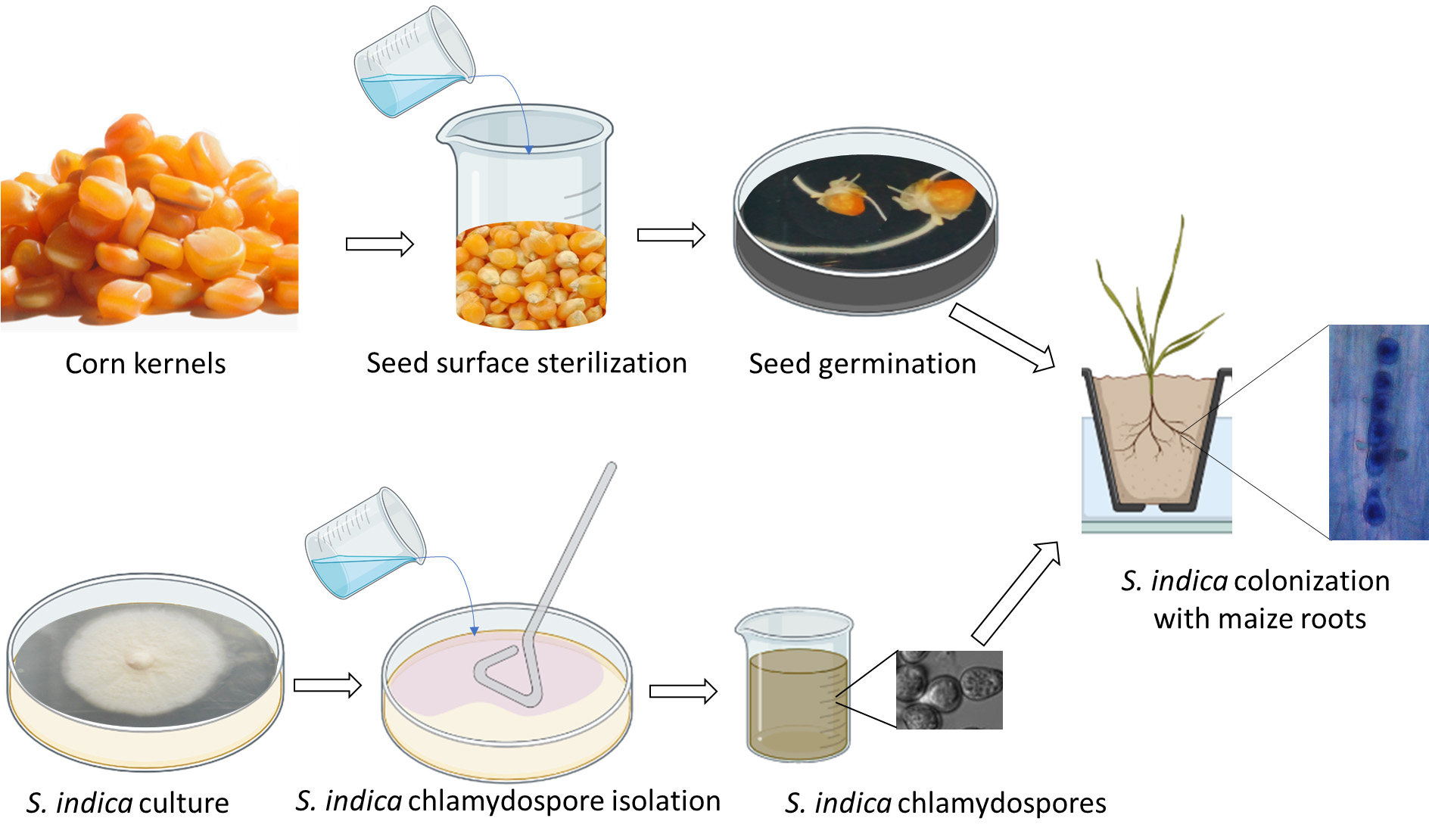
Background
Maize is an important cereal crop and an essential source of food, feed, and biofuel worldwide. However, maize is highly susceptible to biotic and abiotic stresses, such as pests, diseases, and adverse environmental conditions, which can significantly reduce crop yield and quality (Erenstein et al., 2022; Chávez-Arias et al., 2021). Therefore, it is becoming increasingly important to create sustainable and eco-friendly agricultural methods that can improve the productivity and resilience of maize crops. One such strategy is the use of plant growth–promoting fungi (PGPF) to improve plant growth and health (Adedayo and Babalola, 2023).
S. indica (formerly known as Piriformospora indica) is a PGPF that has been reported to enhance the growth and stress tolerance of various plant species, including maize (Singhal et al., 2017; Narayan et al., 2021). The colonization of maize seedlings with S. indica has been shown to increase root growth, nutrient uptake, photosynthetic efficiency, and resistance to various biotic and abiotic stresses (Varma et al., 1999; Narayan et al., 2017, 2021 and 2022; Prasad et al., 2019; Verma et al., 2022). Therefore, the optimization and standardization of the maize seedlings’ colonization protocol with S. indica are crucial to ensure the reproducibility and reliability of the results.
The protocol described in this paper can help explore the mechanisms underlying the beneficial effects of S. indica on maize growth and stress tolerance and identify potential molecular and biochemical markers of plant–fungi symbiosis. Moreover, this protocol can be used to evaluate the performance of different S. indica strains and inoculation methods and to assess the effects of environmental factors on colonization efficiency, and plant growth and development.
Several methodologies have been developed to study the interactions between plant growth–promoting fungi (PGPF) and plants such as wheat (Triticum aestivum), rice (Oryza sativa), barley (Hordeum vulgare), bean (Phaseolus vulgaris), soybean (Glycine max), and chickpea (Cicer arietinum) using S. indica (Rocha et al., 2019; Wahid et al., 2019; El-Maraghy et al., 2020; Mahdi et al., 2022; Verma et al., 2022). One common approach is the use of in vitro culture systems, such as agar dishes or hydroponic cultures, to assess the effects of S. indica on plant growth and stress responses under controlled conditions (Osman et al., 2020). Another approach is the use of field trials to evaluate the performance of S. indica in enhancing crop productivity and quality under natural conditions. Additionally, various molecular and biochemical techniques, such as quantitative polymerase chain reaction (qPCR), metabolomics, and proteomics, were used to analyze the gene and protein expression levels and metabolic pathways involved in the plant–fungi interaction (Gouda et al., 2018).
The protocol described in this paper has several advantages over other published methods (Kumar et al., 2009; Hosseini et al., 2018; Zhang et al., 2018). Firstly, this protocol provides a standardized and reproducible procedure for the colonization of maize seedlings with S. indica. Secondly, this protocol allows for the evaluation of the colonization efficiency and distribution of S. indica in different plant tissues, which provides insights into the spatial and temporal dynamics of the plant–fungi interaction. Thirdly, this protocol can be combined with various physiological, biochemical, and molecular analyses to study the mechanisms underlying the plant–fungi symbiosis.
In addition to the research applications, this protocol has several other possible applications. Firstly, this protocol can be used to develop inoculum production and delivery methods for S. indica, which can be used for commercial purposes, such as biofertilizer or biostimulant production. Secondly, this protocol can be used to screen and identify potential S. indica strains with improved plant growth–promoting and stress tolerance properties, which can be used to develop new and more effective PGPF-based products. Thirdly, this protocol can be used to investigate the interaction between S. indica and other beneficial microbes, such as mycorrhizal fungi or rhizobia, and their combined effects on plant growth and health. Overall, this protocol has the potential to advance research in the field of plant–microbe interactions and to contribute to the development of sustainable and environmentally friendly agricultural practices.
Materials and reagents
Biological materials
Maize seeds (Variety HQPM-5 from Indian Agriculture Research Institute, Pusa, New Delhi, India)
Serendipita indica fungus (strain DSM11827 gifted from Prof. Ajit Verma) (Varma, Kost, Rexer & Franken, 1997, European patent office, Muenchen, Germany. Patent No. 97121440.8-2105, Nov 1998)
Reagents
Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: 1310-58-3)
Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: 7647-01-0)
Phenol (Sigma-Aldrich, catalog number: 108-95-2)
Lactic acid (Sigma-Aldrich, catalog number: 50-21-5)
Glycerol (Sigma-Aldrich, catalog number: 56-81-5)
Trypan blue (Sigma-Aldrich, catalog number: 72-57-1)
Glucose (Sigma-Aldrich, catalog number: 50-99-7)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: 10043-52-4)
Ferrous chloride (FeCl2) (Sigma-Aldrich, catalog number: 7705-08-0)
Calcium nitrate [Ca(NO3)2] (Sigma-Aldrich, catalog number: 13477-34-4)
Magnesium sulfate (MgSO4.7H2O) (Sigma-Aldrich, catalog number: 7487-88-9)
Potassium nitrate (KNO3) (Sigma-Aldrich, catalog number: 7757-79-1)
Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: 7778-77-0)
Boric acid (H3BO3) (Sigma-Aldrich, catalog number: 10043-35-3)
Manganese sulfate monohydrate (MnSO4.H2O) (Sigma-Aldrich, catalog number: 10034-96-5)
Copper sulfate (CuSO4.5H2O) (Sigma-Aldrich, catalog number: 7758-98-7)
Zinc sulfate (ZnSO4.7H2O) (Sigma-Aldrich, catalog number: 7733-02-0)
Ammonium molybdate [(NH4)2MoO4] (Sigma-Aldrich, catalog number: 13106-76-8)
Sodium-hypochlorite (NaClO) (Sigma-Aldrich, catalog number: 7681-52-9)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: 7447-40-7)
Manganese(II) chloride (MnCl2) (Sigma-Aldrich, catalog number: 7773-01-5)
Iron(II) sulfate heptahydrate (FeSO4.7H2O) (Sigma-Aldrich, catalog number: 7782-63-0)
Cobalt(II) chloride (CoCl2) (Sigma-Aldrich, catalog number: 7646-79-9)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: 7647-14-5)
Sucrose (Sigma-Aldrich, catalog number: 57-50-1)
Ethylenediaminetetraacetic acid ferric sodium salt (NaFeEDTA) (Sigma-Aldrich, catalog number: 15708-41-5)
Potassium iodide (KI) (Sigma-Aldrich, catalog number: 7681-11-0)
Manganese(II) chloride (MnCl2) (Sigma-Aldrich, catalog number: 7773-01-5)
Sodium molybdate dihydrate (Na2MoO4.2H2O) (Sigma-Aldrich, catalog number:10102-40-6)
Glycine (Sigma-Aldrich, catalog number: 56-40-6)
Ammonium phosphate dibasic [(NH4)2HPO4] (Sigma-Aldrich, catalog number: 7783-28-0)
Iron(III) chloride (FeCl3) (Sigma-Aldrich, catalog number: 7705-08-0)
Ethanol (Sigma-Aldrich, catalog number: 64-17-5)
Peptone (Sigma-Aldrich, catalog number: 73049-73-7)
Yeast extract (Sigma-Aldrich, catalog number: 8013-01-2)
Casamino acid (Sigma-Aldrich, catalog number: 65072-00-6)
Agar (Sigma-Aldrich, catalog number: 9002-18-0)
BD DifcoTM BactoTM Agar (Sigma-Aldrich, catalog number: DF0140-15-4)
Biotin (Sigma-Aldrich, catalog number: 7646-79-9)
Nicotinamide (Sigma-Aldrich, catalog number: 98-92-0)
Pyridoxal phosphate (Sigma-Aldrich, catalog number: 853645-22-4)
Aminobenzoic acid (Sigma-Aldrich, catalog number: 150-13-0)
Riboflavin (Sigma-Aldrich, catalog number: 83-88-5)
Thiamine hydrochloride (Sigma-Aldrich, catalog number: 67-03-8)
Pyridoxine hydrochloride (Sigma-Aldrich, catalog number: 58-56-0)
Nicotinic acid (Sigma-Aldrich, catalog number: 59-67-6)
Trypticase peptone (Sigma-Aldrich, catalog number: 91079-40-2)
Malt extract (Sigma-Aldrich, catalog number: 8002-48-0)
Myo-Inositol (Sigma-Aldrich, catalog number: 87-89-8)
Solutions
Sterile water (1 L)
Double-distilled water (DI water) (1 L)
Half-strength modified Hoagland solution (1 L) (see Recipes)
Ethanol (1 L) 70% (w/v) (see Recipes)
Sodium hypochlorite (NaClO) solution (1 L) 0.75% (w/v) (see Recipes)
0.8% Bacto agar (500 mL) (see Recipes)
Lactophenol (50%) (see Recipes)
Trypan blue (100 mL) (see Recipes)
10% KOH (100 mL) (see Recipes)
Aspergillus modified medium (AMM) or Kaefer (KF) medium (1 L) (see Recipes)
MN-Agar medium (1L) (see Recipes)
Recipes
Half-strength modified Hoagland solution (1 L)
Reagent Final concentration Quantity Macronutrients Ca(NO3)2·4H2O 4 mM 944.5 mg MgSO4·7H2O 2 mM 492.9 mg KNO3 6 mM 606.6 mg KH2PO4 1 mM 136.08 mg Micronutrients H3BO3 45 μM 2.78 mg MnSO4·4H2O 20 μM 3.01 mg CuSO4·5H2O 0.4 μM 99.87 mg ZnSO4·7H2O 0.7 μM 201.32 mg (NH4)2MoO4·2H2O 0.2 μM 41.18 mg H2O 1,000 mL Total 1,000 mL 70% ethanol (1 L)
Reagent Quantity Ethanol (absolute) 700 mL H2O 300 mL Total 1,000 mL 0.75% sodium-hypochlorite (NaClO) (bleach) solution (100 mL)
Reagent Quantity Sodium hypochlorite solution (6%) 12.5 mL ddH2O 87.5 mL Total 100 mL 0.8% Bacto agar (500 mL)
Reagent Quantity Bacto agar 4g ddH2O 500 mL Total 500 mL Lactophenol (50%)
Reagent Quantity Phenol 150 mL ddH2O 150 mL Lactic acid 125 mL Glycerol 125 mL Total 600 mL Trypan blue (100 mL)
Reagent Quantity Trypan blue 0.1 g Lactophenol 100 mL Total 100 mL 10% KOH (100 mL)
Reagent Quantity KOH 10 g ddH2O 100 mL Total 100 mL Aspergillus modified medium/Kaefer medium (1 L)
Reagent Quantity Glucose 20 g Peptone 2 g Yeast Extract 1 g Casamino acid 1 g Vitamin stock solution 1 mL Macro-element stock solution 50 mL Micro-element stock solution 2.5 mL CaCl2 (0.1 M) 1 mL FeCl2 (0.1 M) 1 mL Agar 10 g pH (Adjust with 1 N HCl) 6.5 ddH2O 944.5 mL Total 1,000 mL Macro-elements stock KCl 10.4 g MgSO4·7H2O 10.4 g KH2PO4 30.4 g ddH2O 1,000 mL Total 1,000 mL Micro-elements stock ZnSO4·7H2O 22 g H3BO3 11 g MnCl2·4H2O 5 g FeSO4·7H2O 5 g CoCl2·6H2O 1.6 g CuSO4·5H2O 1.6 g ddH2O 1,000 mL Total 1,000 mL Vitamin stock Biotin 5 mg Nicotinamide 50 mg Pyridoxal Phosphate 10 mg Aminobenzoic Acid 10 mg Riboflavin 25 mg ddH2O 100 mL Total 100 mL 0.1 M FeCl2 FeCl2 1.62 g ddH2O 100 mL Total 100 mL 0.1 M CaCl2 CaCl2 1.11 g ddH2O 100 mL Total 100 mL MN-Agar medium (1 L)
Reagent Quantity NaCl 23.4 mg KNO3 80 mg Ca(NO3)2·4H2O 288 mg Sucrose 10 g NaFeEDTA 8 mg KI 0.8 mg MnCl2·4H2O 6 mg Na2MoO4·2H2O 0.0024 mg Glycine 3 mg KH2PO4 272.2 mg (NH4)2HPO4 40.8 mg CaCl2 81.6 mg MgSO4·7H2O 731 mg FeCl3 583.9 mg Thiamine hydrochloride 67.4 mg Pyridoxine hydrochloride 67.4 mg Nicotinic acid 0.5 mg Trypticase peptone 1 g Glucose 10 g Malt extract 50 g Myo-Inositol 50 mg Bacto agar 10 g KCl 65 mg H3BO3 1.5 mg MnSO4·H2O 6.0 mg ZnSO4·7H2O 2.7 mg CuSO4·5H2O 0.2 mg pH 5.8 Total 1,000 mL Notes:
All the stock solutions are stored at 4 °C and the vitamin stock at -20 °C. The stock of FeSO4·7H2O is prepared separately.
Filter sterilize the vitamin stocks.
Laboratory supplies
Gloves (Genesee Scientific, catalog number: 44-100M)
Lab coat (Fisher Scientific, catalog number: 19-181-570)
Pipettes of different sizes (Fisher Scientific, catalog number: 13-690-029)
Tip boxes (Fisher Scientific, catalog number: 01-670-713)
1 L Glass bottles (Fisher Scientific, catalog number: 13951L)
100 mL Glass bottles (Fisher Scientific, catalog number: 06-414-1A)
Petri dishes 20mm (Fisher Scientific, catalog number: 08-757-099)
Petri dishes 150mm (Fisher Scientific, catalog number: 50-403-868)
Plastic trays (Fisher Scientific, catalog number: 11-394-455)
Blotting papers (Fisher Scientific, catalog number: 09-301-199)
Germination paper (Fisher Scientific, catalog number: NC1466201)
Spreader (Fisher Scientific, catalog number: 14-665-230)
Beaker (Fisher Scientific, catalog number: 07-250-056)
250 mL flask (Fisher Scientific, catalog number: 10-040F)
Surgical blade (Fisher Scientific, catalog number: 22-079-774)
Muslin cloth ( Amazon.in, item model number: HAZC017639)
Nail paint (Amazon.in, item model number: CC4407)
Equipment
Laminar flow hood (Thermo ScientificTM, catalog number: 1323TS)
Light microscope (Leica Microscope, Type 020-518.500, Germany and Nikon Eclipse Ti)
Autoclave (Thymol autoclave, India, product code: TAI-902)
Incubator shaker (Multitron Incubator Shaker, HT-Infors, Switzerland)
Glass house (School of Life Sciences, Jawaharlal Nehru University, New Delhi India)
Software and datasets
Microsoft Office Excel 10
GraphPad Prism 8
Procedure
Preparation of stock solutions
To ensure the successful execution of all procedures outlined in this protocol and the proper maintenance of the S. indica culture, it is necessary to prepare the listed stock solutions mentioned above, and then prepare working solutions and mixtures in sterile water and store in glass bottles at room temperature, as required during the different steps.
Sterilize the KF medium using the autoclave at 121.6 °C under 15 psi for 20 min before plating.
Maintenance and growth of fungal species (S. indica)
Prepare 25 mL of KF-agar medium in a 100 mL glass bottle and autoclave it.
Add the vitamin stock into this KF-agar medium at a temperature of 40 °C (kept in a water bath) and mix gently to avoid bubble formation.
Pour this KF-agar medium into a 90 mm Petri dish in a laminar flow hood.
Allow 20–30 min to complete the solidification of the KF media in the Petri dish inside the laminar flow hood. Note: To improve solidification and prevent water droplet accumulation inside the lids, it is recommended to keep the Petri dish lid open.
Inoculate a single ball/colony of S. indica from the liquid KF medium (the sample has already been incubated in a shaker incubator at 30 ± 2 °C for 7–9 days with shaking at 100 rpm) or cut a slice sized 5 × 5 mm of pregrown S. indica on a solid dish culture using a sterilized surgical blade and place this slice of KF-agar-containing S. indica culture on the center of the 90 mm fresh KF medium Petri dish.
Note: A fine pressure can be applied onto the slice to make sure the S. indica slice can make full contact with the fresh KF medium.
Incubate the dishes at 28–30 °C incubator in darkness for 5–7 days for full growth.
After incubation, observe the growth of the S. indica by visualizing the grey-colored, cotton-like growth (see the right panel of Figure 1).
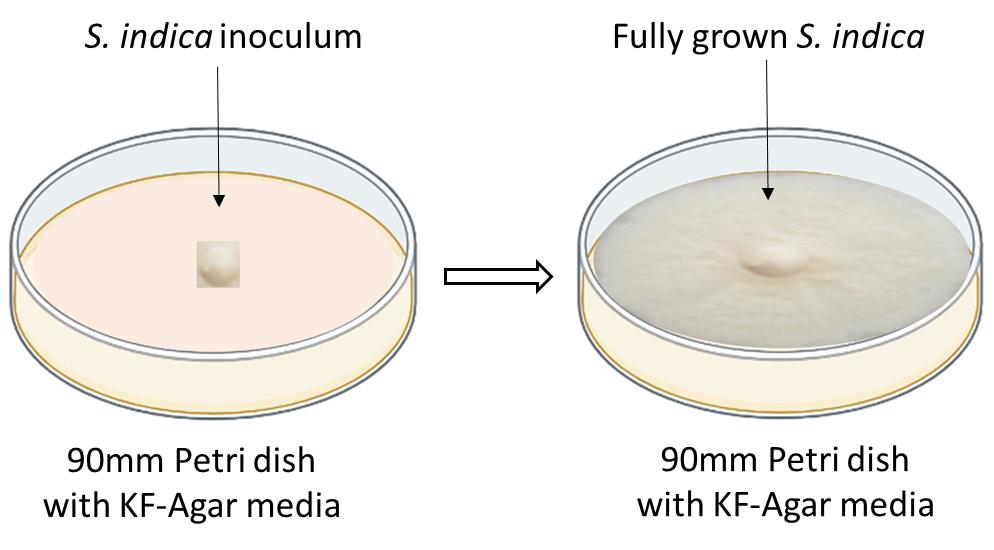
Figure 1. Schematics showing how to inoculate the S. indica on a solid KF-agar 90 mm Petri dishNow, S. indica is ready for the preparation of chlamydospores and further colonization study with maize seedlings.
Note: For liquid broth medium growth, S. indica was maintained routinely on solidified Aspergillus (Aspergillus niger) modified medium (Hill and Kafer, 2001). Growth of S. indica was studied in 250 mL culture flasks with constant shaking at 100 rpm, and at 30 ± 2 °C for 7–9 days in a metabolic shaker (Multitron Incubator Shaker, HT-Infors, Switzerland).
Maize seed surface sterilization and germination
Take the required quantity of desired functional maize seeds (we used the HQPM5 variety from IARI, New Delhi, India).
Take approximately 120 seeds (in our case) and dip them into 100 mL of autoclaved DD water for 1 h using a 250 mL beaker.
Wash the maize seeds with detergent (teepol or Triton X-100) using 3–5 drops in 50 mL of water.
Repeat washing of the maize seeds with autoclaved DD water by changing the water approximately 5 times for a total of 10 min.
Sterilize the maize seeds by soaking them in a 250 mL beaker containing 70% ethanol for 40 s, manually swirling it.
Remove the ethanol and wash five times with autoclaved DD water for a total of 10 min.
Again, sterilize the maize seeds by soaking them in a 100 mL beaker containing 50 mL of 6% NaOCl (final concentration 0.75%) for 1 min, manually swirling it.
Wash the maize seeds with autoclaved DD water six times for 10 min.
Note: In this final washing, make sure there is no remaining NaOCl.
Transfer the maize seeds into a new beaker and heat it in a water bath at 60 °C for 5 min.
Place the maize seeds in water agar dishes/germination sheets using sterile forceps, spacing them 1 inch apart for germination at 28 °C in the dark for 3 days (10–15 seeds per dish).
Note: Use bi size (150 mm) glass or plastic Petri dishes for making 0.8% water agar dishes (0.8% Bacto Agar, Difco, Detroit, MI, USA).
Preparation of chlamydospores of S. indica
Take the fully grown S. indica KF dishes (6–10 days old) to prepare the chlamydospores.
Note: Use the completely grown S. indica on a KF medium and open the dish in laminar air flow.
Take approximately 2–3 mL of 2% filter-sterilized glucose solution prepared in DD water and pour on the top surface of the fully grown S. indica KF dishes.
Spread the 2% glucose solution gently using a sterile plastic spreader and aim to just collect the chlamydospores in the solution phase.
Note: Try not to disturb the bottom part of the S. indica-containing hyphae.
Collect the solution containing chlamydospores in 2 mL Eppendorf tubes.
Filter this solution using a sterile muslin cloth to remove the fungal hyphae and any part of solid medium, transferring the chlamydospores to another tube.
Centrifuge the S. indica spores (in Eppendorf tubes) at 3× g at 30 °C for 3–4 h.
The quality of the spores can be checked by observation under a light microscope using glass slides.
The number of S. indica chlamydospores can be maintained at 5 × 105/mL using a hemocytometer for further colonization with maize seedlings.
Colonization of S. indica with maize plants
Take out the germinated maize seedlings from the water agar dishes or germination sheets (3–4 days old) and wash the roots with sterilized water, then dip the roots in S. indica chlamydospore suspensions (maintained at 5 × 105 spores per mL) to start the inoculation.
Note: Forceps can be used to dip the roots into the S. indica chlamydospore suspensions.
Transfer the inoculated maize seedlings with the chlamydospores of S. indica to the sterile MN-agar medium.
Grow the inoculated maize seedlings in the MN medium for a minimum of 7 days for colonization.
Alternatively, grow the inoculated maize seedlings in pots filled with a mixture of sterile sand and soil in the ratio of 3:1.
Grow the inoculated plants under controlled conditions in a glasshouse with an 8 h light (1,000 Lux)/16 h dark period at a temperature of 28 °C with a relative humidity of 60%–70%.
Supplement the plants weekly with half-strength modified Hoagland solution.
For control plants (without S. indica inoculated), mock inoculate with autoclaved DD water–mixed sand. Check the S. indica colonization at different time points under the light microscope. In our case, plant roots were harvested at different dpi (5, 10, 15, and 20 days) and were assessed for colonization by trypan blue staining as shown below.
Histochemical analysis
Harvest the plant roots at 5, 10, 15, and 20 days after inoculation, and choose approximately 10–20 random samples of the root system for colonization estimation.
Note: Use small surgical scissors to cut the root sample from the maize plants.
To soften the root samples, treat them with 10% KOH solution for 15 min and then acidify them with 1 N HCl for 10 min. Finally, stain them with 0.02% trypan blue overnight (Narayan et al., 2021).
Samples were destained with 50% Lactophenol for 1–2 h. Keep the samples on a rotary shaker.
Cut the root samples into 1 cm small fragments using the surgical blade and mount them on glass slides.
Note: Label the glass slides with sample number and root number (see Figure 2).
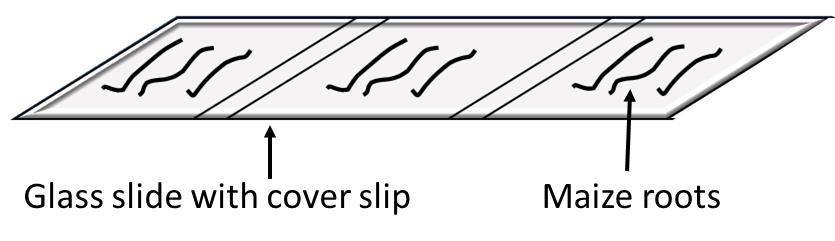
Figure 2. Schematics showing a glass slide mounted with small fragments of the S. indica-colonized maize roots after the trypan blue staining, for microscopic studyCover the root samples with the glass coverslips, pressing the coverslips gently to crush the roots, and seal slides using nail paint (see Figure 2)
Observe these slides under a light microscope to see the blue-stained and pear-shaped chlamydospores of S. indica under the different magnifications. Please see Figure 3 to see how chlamydospores of S. indica look like in a colonized state inside the root cortical cells, under light microscope observation.
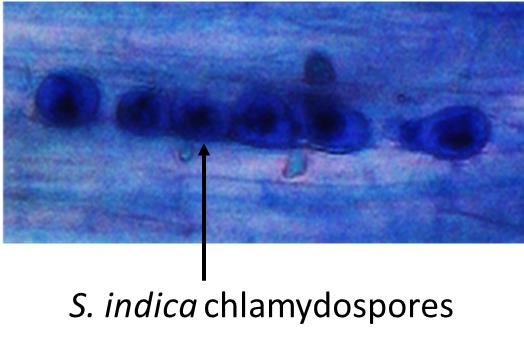
Figure 3. Blue-colored chlamydospores of S. indica inside the maize root cortical cells, viewed under a light microscope.Count the colonized root segments for further calculation.
The distribution of chlamydospores within the root was taken as an index for studying colonization. Percent colonization was calculated for the inoculated plants according to the method described previously (Narayan et al., 2017 and 2021). Specifically, it was calculated using the following formula. The calculation involved the total number of randomly selected roots, the number of colonized root segments observed under a microscope, and the total number of root segments taken from a random sample to observe chlamydospores under a microscope. Both biological and technical repeats were combined to calculate the standard deviation and determine the significance of the data. Table 1 provides an example of percent colonization.
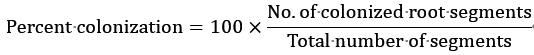
Table 1. Percent colonization of S. Indica with maize plant root
Time after inoculation (days) Percent colonization (%) 5 7.5 ± 5 10 27.5 ± 9.6 15 45 ± 12.9 20 70 ± 11.5
Data analysis
Data can be analyzed using Excel. In our case, we used three biological and three technical repeats. Each time we used 120 seeds of maize. Statistical tests like standard deviation and standard error analysis can be done by using sigma plot or GraphPad Prism 8.
Validation of protocol
This protocol or parts of it has been used and validated in the following research article(s):
Kundu et al. (2022). Piriformospora indica recruits host-derived putrescine for growth promotion in plants. Plant Physiology. (Figure 6, panel h).
Narayan et al. (2021). Sulfur transfer from the endophytic fungus Serendipita indica improves maize growth and requires the sulfate transporter SiSulT. Plant Cell. (Figure 1, panel c).
Verma et al. (2022). Functional characterization of a high‐affinity iron transporter ( PiFTR ) from the endophytic fungus Piriformospora indica and its role in plant growth and development. Environmental Microbiology. (Figure 6, panel c).
Narayan et al. (2017). Antioxidant enzymes in chickpea colonized by Piriformospora indica participate in defense against the pathogen Botrytis cinerea. Scientific Reports. (Figure 1).
Jogawat et al. (2013). Piriformospora indica rescues growth diminution of rice seedlings during high salt stress. Plant signaling & behavior. (Figure 1, panel b).
Kumar et al. (2009). Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology. (Figure 1).
General notes and troubleshooting
General notes
The colonization efficiency of S. indica may vary depending on the maize cultivar, growth conditions, and chlamydospore quality.
Overstaining can hide the chlamydospores with the root tissues.
To improve colonization efficiency, use a fresh culture of S. indica.
Limitations associated with this protocol:
The colonization efficiency of S. indica may vary depending on the maize cultivar, growth conditions, and inoculation methods, which may affect the reproducibility of the results.
The use of high-throughput molecular techniques, such as RNA sequencing or metabolomics, may require a large amount of plant tissue, which may limit the application of this protocol to small-scale experiments.
This protocol only focuses on the early stages of maize growth, and the long-term effects of S. indica on plant growth and stress tolerance are still unclear.
Troubleshooting (Table 2)
Table 2. Troubleshooting
| Common problems | Troubleshooting |
| Low colonization efficiency | Check the viability of the chlamydospores by growing on a liquid broth or solid KF-agar dishes and compare with normal S. indica growth. |
| Low germination efficiency | Check the quality of seeds. Avoid using very old, dormant seeds. |
| Chlamydospores are not visible in the microscope | Stain chlamydospores with trypan blue for a longer time. |
| Blue-colored chlamydospores are hidden in root tissues | Try to destain the roots for a longer time using Lactophenol. |
| Contamination of other fungal species | Ensure all equipment and reagents are in sterile conditions. |
Acknowledgments
Jawaharlal Nehru University, New Delhi, and University Grants Commission, New Delhi India, ICMR, New Delhi, India is highly acknowledged for the financial support. We also acknowledge the original research paper in which this protocol was described and validated was published by Narayan et al., 2021, The Plant Cell (doi: 10.1093/plcell/koab006) from School of Life Sciences, Jawaharlal Nehru University, New Delhi, India.
Competing interests
All the authors declare no conflict of interests.
References
Adedayo, A. A. and Babalola, O. O. (2023). Fungi That Promote Plant Growth in the Rhizosphere Boost Crop Growth. J. Fungi 9(2): 239.
- Chávez-Arias, C. C., Ligarreto-Moreno, G. A., Ramírez-Godoy, A. and Restrepo-Díaz, H. (2021). Maize Responses Challenged by Drought, Elevated Daytime Temperature and Arthropod Herbivory Stresses: A Physiological, Biochemical and Molecular View. Front. Plant Sci. 12: e702841.
- El-Maraghy, S. S., Tohamy, T. A. and Hussein, K. A. (2020). Role of plant-growth promoting fungi (PGPF) in defensive genes expression of Triticum aestivum against wilt disease. Rhizosphere 15: 100223.
- Erenstein, O., Jaleta, M., Sonder, K., Mottaleb, K. and Prasanna, B. (2022). Global maize production, consumption and trade: trends and R&D implications. Food Secur. 14(5): 1295–1319.
- Gouda, S., Kerry, R. G., Das, G., Paramithiotis, S., Shin, H. S. and Patra, J. K. (2018). Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 206: 131–140.
- Hill, T. W. and Kafer, E. (2001). Improved protocols for Aspergillus minimal medium: trace element and minimal medium salt stock solutions. Fungal Genet Rep. 48(1): 20–21.
- Hosseini, F., Mosaddeghi, M. R., Dexter, A. R. and Sepehri, M. (2018). Maize water status and physiological traits as affected by root endophytic fungus Piriformospora indica under combined drought and mechanical stresses. Planta 247(5): 1229–1245.
- Kumar, M., Yadav, V., Tuteja, N. and Johri, A. K. (2009). Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology 155(3): 780–790.
- Mahdi, L. K., Miyauchi, S., Uhlmann, C., Garrido-Oter, R., Langen, G., Wawra, S., Niu, Y., Guan, R., Robertson-Albertyn, S., Bulgarelli, D., et al. (2022). The fungal root endophyte Serendipita vermifera displays inter-kingdom synergistic beneficial effects with the microbiota in Arabidopsis thaliana and barley. ISME J. 16(3): 876–889.
- Narayan, O. P., Kumar, P., Yadav, B., Dua, M. and Johri, A. K. (2022). Sulfur nutrition and its role in plant growth and development. Plant Signal. Behav.: e2030082.
- Narayan, O. P., Verma, N., Jogawat, A., Dua, M. and Johri, A. K. (2021). Sulfur transfer from the endophytic fungus Serendipita indica improves maize growth and requires the sulfate transporter SiSulT. Plant Cell 33(4): 1268–1285.
- Narayan, O. P., Verma, N., Singh, A. K., Oelmüller, R., Kumar, M., Prasad, D., Kapoor, R., Dua, M. and Johri, A. K. (2017). Antioxidant enzymes in chickpea colonized by Piriformospora indica participate in defense against the pathogen Botrytis cinerea. Sci. Rep. 7(1): 13553.
- Osman, M., Stigloher, C., Mueller, M. J. and Waller, F. (2020). An improved growth medium for enhanced inoculum production of the plant growth-promoting fungus Serendipita indica. Plant Methods 16(1): 1–7.
- Prasad, D., Verma, N., Bakshi, M., Narayan, O. P., Singh, A. K., Dua, M. and Johri, A. K. (2019). Functional Characterization of a Magnesium Transporter of Root Endophytic Fungus Piriformospora indica. Front. Microbiol. 9: e03231.
- Rocha, I., Duarte, I., Ma, Y., Souza-Alonso, P., Látr, A., Vosátka, M., Freitas, H. and Oliveira, R. S. (2019). Seed Coating with Arbuscular Mycorrhizal Fungi for Improved Field Production of Chickpea. Agronomy 9(8): 471.
- Singhal, U., Prasad, R. and Varma, A. (2017). Piriformospora indica (Serendipita indica): The Novel Symbiont. Mycorrhiza - Function, Diversity, State of the Art: 349–364.
- Varma, A., Verma, S., Sudha, Sahay, N., Bütehorn, B. and Franken, P. (1999). Piriformospora indica, a Cultivable Plant-Growth-Promoting Root Endophyte. Appl. Environ. Microbiol. 65(6): 2741–2744.
- Verma, N., Narayan, O. P., Prasad, D., Jogawat, A., Panwar, S. L., Dua, M. and Johri, A. K. (2022). Functional characterization of a high‐affinity iron transporter ( PiFTR ) from the endophytic fungus Piriformospora indica and its role in plant growth and development. Environ. Microbiol. 24(2): 689–706.
- Wahid, F., Sharif, M., Fahad, S., Adnan, M., Khan, I. A., Aksoy, E., Ali, A., Sultan, T., Alam, M., Saeed, M., et al. (2019). Arbuscular mycorrhizal fungi improve the growth and phosphorus uptake of mung bean plants fertilized with composted rock phosphate fed dung in alkaline soil environment. J. Plant Nutr. 42(15): 1760–1769.
- Zhang, W., Wang, J., Xu, L., Wang, A., Huang, L., Du, H., Qiu, L. and Oelmüller, R. (2018). Drought stress responses in maize are diminished byPiriformospora indica. Plant Signal. Behav. 13(1): e1414121.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Narayan, O. P., Yadav, B., Verma, N., Dua, M. and Johri, A. K. (2023). Maize Seedlings Colonization with Serendipita indica and Its Colonization Efficiency Analysis. Bio-protocol 13(20): e4855. DOI: 10.21769/BioProtoc.4855.
Category
Plant Science > Plant immunity > Host-microbe interactions
Plant Science > Plant physiology > Biotic stress
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link