- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Production, Extraction, and Solubilization of Exopolysaccharides Using Submerged Cultures of Agaricomycetes
Published: Vol 13, Iss 19, Oct 5, 2023 DOI: 10.21769/BioProtoc.4841 Views: 2206
Reviewed by: Lucy XieOsarenkhoe Omorefosa OsemwegieAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Chitin Extraction and Content Measurement in Magnaporthe oryzae
Xinyu Liu and Zhengguang Zhang
Mar 5, 2017 9067 Views

Analysis of Lipid-linked Oligosaccharides Synthesized in vivo in Schizosaccharomyces pombe
Ayelen Valko [...] Cecilia D’Alessio
Sep 20, 2022 2244 Views

Surface Plasmon Resonance for the Interaction of Capsular Polysaccharide (CPS) With KpACE
Zhe Wang [...] Chao Cai
Jun 20, 2025 3575 Views
Abstract
Macrofungi, also known as mushrooms, can produce various bioactive compounds, including exopolysaccharides (EPS) with distinct biological properties and subsequent industrial applications in the preparation of cosmetics, pharmaceuticals, and food products. EPS are extracellular polymers with diverse chemical compositions and physical properties secreted by macrofungi in the form of capsules or biofilms into the cellular medium. Submerged cultivation is an industrially implemented biotechnological technique used to produce a wide variety of fungal metabolites, which are of economic and social importance due to their food, pharmaceutical, and agronomic applications. It is a favorable technique for cultivating fungi because it requires little space, minimal labor, and low production costs. Moreover, it allows for control over environmental variables and nutrient supply, essential for the growth of the fungus. Although this technique has been widely applied to yeasts, there is limited knowledge regarding optimal growth conditions for filamentous fungi. Filamentous fungi exhibit different behavior compared to yeast, primarily due to differences in cell morphology, reproductive forms, and the type of aggregates generated during submerged fermentation. Furthermore, various growing conditions can affect the production yield of metabolites, necessitating the development of new knowledge to scale up metabolite production from filamentous fungi. This protocol implements the following culture conditions: an inoculum of three agar discs with mycelium, agitation at 150 rpm, a temperature of 28 °C, an incubation time of 72 h, and a carbon source concentration of 40 g/L. These EPS are precipitated using polar solvents such as water, ethanol, and isopropanol and solubilized using water or alkaline solutions. This protocol details the production procedure of EPS using submerged culture; the conditions and culture medium used are described. A detailed description of the extraction is performed, from neutralization to lyophilization. The concentrations and conditions necessary for solubilization are also described.
Key features
• Production and extraction of EPS from submerged cultures of mycelial forms of macrofungi.
• Modification of the method described by Fariña et al. (2001), extending its application to submerged cultures of mycelial forms of the macrofungi.
• Determination of EPS production parameters in submerged cultures of mycelial forms of macrofungi.
• EPS solubilization using NaOH (0.1 N).
Graphical overview
Background
Macrofungi are fungi that produce reproductive bodies that are visible to the naked eye. Some macrofungi known for their production of polysaccharides are the Shiitake (Lentinula eodes) or the mushroom turkey tail (Trametes versicolor). Macrofungal polysaccharides are a group of compounds of great interest in bioprospecting studies of both medicinal and edible fungi, owing to their broad spectrum of bioactivities, including cytotoxic, antiproliferative, antitumor, immunomodulatory, anti-inflammatory, antioxidant, and stimulatory effects, on the colon microflora (Arora and Tandon, 2015; X. Meng, 2016; Nowakowski et al., 2021). These bioactivities are related to the chemo-preventive action of macrofungal polysaccharides against different types of cancers such as colorectal cancer and other chronic diseases. Macrofungi polysaccharides are complex carbohydrates found in the cell walls and extracellular matrices. These polysaccharides play a crucial role in the structural integrity and functionality of fungal organisms. They can be homopolysaccharides that contain repeating units of the same monosaccharide or heteropolysaccharides, containing several types of monosaccharides. Polysaccharides can also include other groups, such as sulfate or glucuronate, and adopt simple, triple, or random helical conformations depending on the conditions of the culture medium (Huang and Nie, 2015; Gong et al., 2020), significantly affecting their solubility in water or marginally basic aqueous solutions. Solubility and the type of conformation adopted are crucial for biological activity and must be controlled in different assays at the in vitro level because of their effects on the expected results. Structural conformation is also responsible for the great water solubility of polysaccharides: for example, scleroglucan secreted by Sclerotium fungi adopts a highly ordered, rigid, triple helical tertiary structure when dissolved in water at room temperature and low concentrations of alkali, usually below 0.15 M NaOH (Castillo et al., 2015). Water solubility of fungal exopolysaccharides (EPS) depends, to a large extent, on monosaccharide composition and structure and size of the respective molecules (Jaros et al., 2018). These variables, together with differences in the type of bonding and branching, tertiary structure, electrical charge and conformation, and solubility generate a diversity of physical and chemical properties, as well as reported biological activities (Bacic et al., 2009).
Polysaccharides produced by macrofungi can be classified into three main groups based on their location in the cell: (1) cytosolic polysaccharides that provide a source of carbon and energy for the cell; (2) cell wall polysaccharides that include the peptidoglycans, teichoic acids, and lipopolysaccharides; and (3) extracellular polysaccharides that are exuded in the form of capsules or biofilms, also known as EPS, which constantly diffuse into the cell wall and cell cultures (Kambourova et al., 2015; Wang et al., 2022). For the production of EPS, the submerged culture technique is used at both laboratory and industrial scales, where the mycelium of the fungus grows while floating as small spheres called pellets when there is agitation (Papagianni, 2004). The submerged fermentation process is widely used for producing different types of metabolites on a large scale or under flask conditions in which organisms can be obtained in limited physical spaces under conditions controlled and optimized for the production of biomass; another advantage is the ease of separating the mycelium from the EPS in the culture medium, obtaining a product of uniform quality for their purification in a further step. On the other hand, it provides several advantages such as higher productivity and yields, lower labor costs, and lower contamination risk. Submerged culture offers the potential advantages of faster production of EPS within a smaller space, reduced possibility of contamination, and better control of cultivation conditions such as carbon source and temperature (Dudekula et al., 2020).
In addition to submerged cultures, several other methods have been employed for the production of EPS. Some of these methods are: (1) surface culture, in which the microorganisms are grown on the surface of a solid substrate, such as agar or other suitable media; the EPS is produced and secreted by the microorganisms onto the surface of the substrate (Breitenbach et al., 2022); (2) solid-state fermentation, which involves the growth of microorganisms on solid substrates without the addition of free-flowing water; the microorganisms utilize the nutrients present in the solid substrate to produce EPS; common solid substrates used include agricultural residues, cereal grains, and sawdust (Isikhuemhen et al., 2014); (3) biofilm reactors, which provide an ideal environment for the growth of biofilms, allowing the microorganisms to attach to a surface and form a structured matrix; EPS production occurs with the biofilm structure (Seneviratne et al., 2008); (4) immobilized cell systems, in which the microbial cells are immobilized or attached to a solid support matrix, such as alginate beads, polyurethane foam, or glass beads; the immobilized cells produce EPS while attached to the support matrix, allowing for easy separation and recovery of the EPS (Li et al., 2017); and (5) membrane bioreactors, which combine the use of submerged culture with the retention of microbial cells and EPS using a membrane filtration system; the membrane allows the culture medium to pass through while retaining the microbial cells and EPS, which can then be harvested and recovered (Brito et al., 2019). It is important to note that the method of choice for EPS production depends on various factors, including the microorganism used, the type of EPS desired, and the scale of production. Different methods offer distinct advantages and disadvantages in terms of yield, productivity, cost, and ease of operation.
Extensive investigations have been conducted to enhance the yield of EPS production. These studies have focused on optimizing various factors, including the assessment of different carbon sources (e.g., glucose, sucrose, fructose) and nitrogen sources (e.g., peptone, yeast extract, ammonium nitrate). The influence of pH levels and incubation time on scleroglucan production has also been thoroughly examined. Notably, Fariña et al. (2001) successfully identified the optimal conditions for scleroglucan production by Sclerotium rolfsii. Their research determined the most advantageous carbon and nitrogen sources, optimal pH range, and incubation time, resulting in significant improvements in scleroglucan yields. Our approach differs from the method described by Fariña et al. (2001), as we evaluate the impact of two carbon sources. Moreover, we consider the contribution of the mushroom species as a crucial factor in EPS production. Incorporating a comprehensive range of tests during initial evaluation enables the identification of optimal strains for bioactive EPS production (Mahapatra and Banerjee, 2013; Angelova et al., 2022). Among agaricomycetes, Agaricales and Polyporales are the two most prominent and extensively used orders in biotechnology. To validate this protocol, representative strains from Agaricales and Polyporales were selected, namely Schizophyllum radiatum and Lentinus crinitus, respectively.
Materials and reagents
Petri dishes (90 mm × 15 mm) (Nest, catalog number: 752001)
Polypropylene microcentrifuge tubes (2.0 mL) (Eppendorf, Biologix, catalog number: 80-0020)
Paper towel
Conical tubes (50 mL) (Falcon, catalog number: 602002-1)
Erlenmeyer flask (250 mL)
Beakers (10 and 500 mL) (Glassco Aleman, catalog number: 229.202.02, 229.202.08A)
Borosilicate glass bottle with screw cap with rubber/Teflon liner (250 mL) (Glassco, catalog number: 6679.269.245.01)
Nichrome inoculating needle (Citoplus, catalog number: 33210001B)
Filter paper, pore size: 3 μm (Munktell Ahlstrom, catalog number: 391)
Filter paper, pore size: 10 μm (Ahlstrom Munksjo, catalog number: 389)
Pistil (Axygen, Pes15b)
Core samplers (diameter: 5 mm)
Magnetic stir bar (KartellTM, catalog number: 0076800)
Biological materials
Schizophyllum radiatum Fr. (Fungario Universidad del Tolima; Laboratory collection, strain 030, voucher: LRD27)
Lentinus crinitus (L.) Fr. (Fungario Universidad del Tolima; Laboratory collection, strain 147, voucher: ZF37)
Reagents
Agar agar (Sigma-Aldrich, catalog number: BCBS4444V, 05040)
Peptone (EMD, Millipore, catalog number: WM879031-906BD)
Yeast extract (OXOID, catalog number: LP0021)
Malt extract (OXOID, catalog number: LP0039)
D-Glucose (EMD Millipore, catalog number: K51794437 004)
D-Sucrose (Fisher Scientific, catalog number: S5-500)
Sodium hydroxide (NaOH) (EMD Millipore, catalog number: B1101198-436)
Magnesium sulfate heptahydrate (MgSO4·7H2O) (Honeywell Specialty Chemicals Seelze, catalog number: 10314903)
Iron (II) sulfate heptahydrate (FeSO4·7H2O) (Sigma-Aldrich, catalog number: F7002)
Ammonium sulfate [(NH4)2SO4] (PanReac Química SLU, catalog number: A1032)
Anhydrous dipotassium hydrogen phosphate (K2HPO4) (Mallinckrodt Baker, catalog number: 3252-01)
Ethanol, 96% (Fábrica de Licores Tolima)
Solutions
Malt agar medium (see Recipes)
SYAMI culture medium (see Recipes)
Sodium hydroxide (NaOH) 0.1 N (see Recipes)
Recipes
Malt agar medium
Reagent Final concentration Peptone 3 g/L Malt extract 30 g/L Agar-agar 15 g/L SYAMI culture medium
Reagent Final concentration MgSO4·7H2O 0.05 g/L K2HPO4 2.0 g/L FeSO4·7H2O 0.2 g/L (NH4)2SO4 0.15 g/L Yeast extract 2.0 g/L Carbon source (glucose or sucrose) 40 g/L Sodium hydroxide (NaOH) 0.1 N
Reagent Quantity NaOH 1 g H2O 250 mL
Equipment
Centrifuge (HERMLE Labortechnik GmbH, Z326K, catalog number: 311)
Orbital shaker (IKA LABORTECHNIK, KS250 basic)
Fume hood (Jpinglobal, catalog number: JPCEGH1200-BB-PP)
Laminar flow cabinet, horizontal (Purificación y análisis de fluidos)
Ultra-low temperature freezer, -85 °C (Kaltis, catalog number: 390)
Freeze dryer (Buchi, model: Lyovapor L-200)
Autoclave (All American, model: 1941X)
Refrigerator (COLDLINE, model: FORTE V13-RHC)
Barnstead Smart2Pure UV/UF (Thermo Scientific, type: Smart2Pure 3 UV/UF)
Hot plate magnetic stirrer (Stuart, catalog number: UC152)
Analytical balance (KERN, type: ACS 220-4)
Blender (Recco, model: 242748)
Drying stove (Memmert, catalog number: ref 1470)
Desiccator (Metacrilato 250 mm)
Sieve (Endecotts Ltd., ASTM E-11, catalog number: 65803- TAN AC8-1-1/2, 37,3)
Borosilicate glass filtration system (Glassco, catalog number: COL-764-260.202.01)
Incubator, 28 °C (Binder, catalog number: BD023UL)
pH meter (SI Analytics, type: HandyLab 100)
Water bath (Buchi, catalog number: B-480)
Software and datasets
Microsoft Excel for calculations
RStudio Team, 2015. RStudio: Integrated Development Environment for R. Boston, MA. Available at: http://www.rstudio.com/
Procedure
Production of EPS using submerged cultures
S. radiatum and L. crinitus culture preparation.
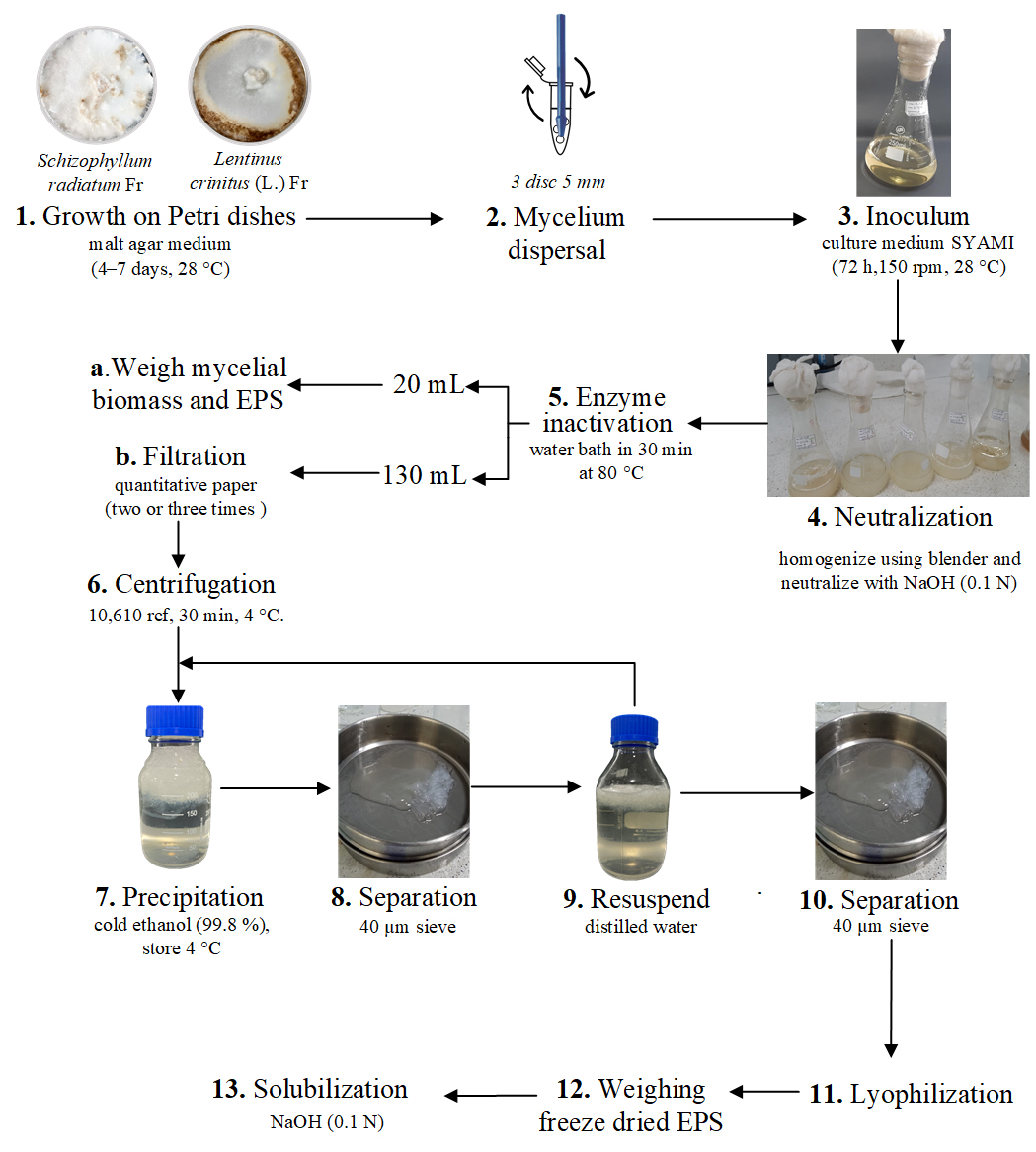
Inoculate each fungal strain from a 4 °C distilled water stock onto solid malt agar medium in a 90 mm × 15 mm Petri dish and incubate at 28 °C until the plate attains full growth for 4–7 days (see Graphical overview, step 1). It is also possible to use other Petri dish sizes.
After full growth, cut the solid malt agar medium with a sterile core sampler (5 mm diameter) and transfer three discs of each fungal strain to 2.0 mL polypropylene microcentrifuge tubes, using a sterile nichrome inoculating needle.
Add 1 mL of sterile distilled water and disperse the agar discs using a pistil. Another option to disperse is to use a stainless-steel immersion blender for 3 s.
Transfer the mixture to a 250 mL Erlenmeyer containing 50 mL of SYAMI culture medium previously autoclaved at 15 psi for 15 min and incubate by shaking at 150 rpm and 28 °C for 72 h or another specific duration; this time depends on the experimental design used and the study strain. For example, S. radiatum strain can produce EPS at 72 h and its maximum production is reached at 144 h. L. crinitus strain can produce EPS at 144 h. Other fungal spices may require more time for EPS production.
Note: Work under laminar flow cabinet.
EPS extraction
Neutralization and enzyme inactivation.
After the incubation time, add the culture to a 500 mL beaker and measure the pH. The pH can be between 4 and 5.
Add sterile distilled water in a 2:1 ratio (100 mL of H2O to 50 mL of culture).
Homogenize the cultures (mycelia and liquid) using immersion blender (two pulses).
Neutralize the mixture (pH = 7.0) using NaOH (0.1 N).
Transfer the mixture to a 250 mL borosilicate glass bottle and incubate in a water bath for 30 min at 80 °C.
Note: Work under fume hood.
Weighing mycelial biomass and EPS.
Place 20 mL of the mixture in 50 mL conical tubes. In the following steps, the weight of the mycelial biomass and EPS will be determined.
Centrifuge at 10,610× g for 30 min at 4 °C.
Transfer the supernatant to a fresh 50 mL conical tube. Add cold ethanol (99.8%) to the supernatant in a 1:1 ratio for EPS precipitation. Store this mixture and biomass pellet at 4 °C for 12 h.
Transfer the precipitated EPS and biomass pellets obtained by centrifugation to quantitative filter paper discs and place them on 100 mm × 15 mm Petri dishes that were previously weighed. Place each sample on a different filter/Petri dish.
Incubate the Petri dishes in a drying stove at 100–110 °C for 2 h.
Place the Petri dishes in a desiccator for 30 min.
Subsequently, weigh the Petri dishes for each sample (EPS precipitated and biomass pellet).
Use the following formula to calculate the dry weight of biomass and EPS:
Dry weight = (C - A)/(B - A) × 100
where A is the weight of the empty filter paper/Petri dish, B is the weight of the filter paper/Petri dish with the fresh sample, and C is the weight of the filter paper/Petri dish with the dried sample.
EPS purification.
Filter 130 mL of the mixture derived from step B1e using a quantitative paper (pore size: 10 μm). Use a borosilicate glass filtration system.
Repeat the filtering step twice using a quantitative paper (pore size: 3 μm). Use a borosilicate glass filtration system. This prevents the passage of small fragments of mycelium.
Transfer the mixture to 50 mL conical tubes.
Centrifuge at 10,610× g for 30 min at 4 °C.
Transfer the supernatant to a 500 mL borosilicate glass bottle.
Add cold ethanol (96%) in a 1:1 ratio for EPS precipitation. Incubate at 4 °C for 12 h (Figure 1a).

Figure 1. Precipitation, lyophilization, and solubilization of exopolysaccharides (EPS) obtained from submerged cultures of macrofungiSeparate the EPS using a 40 μm sieve. The EPS will be suspended on top of the sieve. Use a container under the sieve to collect the ethanol (see Graphical overview, steps 8, 9, and 10). It is also possible to use filter paper of the same pore size.
Add sterile distilled water and repeat steps B3f and B3g.
Place the obtained EPS in an ultra-low temperature freezer (-85 °C, 2 h).
Freeze-dry the EPS (see Table 1).
Table 1. Parameters for lyophilization of macrofungi exopolysaccharides (EPS)
Dried material Strains Freezing parameters Drying shelf temperature regulation Pressure of the chamber Drying time EPS S. radiatum and L. crinitus -50 °C up to 60 °C 0.01 mbar 72 h Determine the weight of the freeze-dried EPS (Figure 1b).
Solubilization
Preparation of concentrated EPS solution.
Weigh 100 mg of lyophilized EPS in a 50 mL beaker.
Add 1.25 mL of NaOH (0.1 N) and 10 mL of sterile distilled water.
Dissolve the mixture using a magnetic stirrer for 1–2 h. Do not heat.
Add sterile distilled water to a final volume of 25 mL.
The final concentration is 4 mg/mL.
The final concentration of NaOH is 0.005 N.
You can prepare solutions at different concentrations as required by assays.
Consider the volume of NaOH (0.1 N) used because it can modify the EPS structure. In this case, it is recommended to use the smallest volume possible and not exceed the final concentration of 0.005 N.
The prepared EPS solution can be stored at 4 °C (Figure 1c).
Data analysis
Establish an experimental design for EPS production using a submerged culture. Use a central or factorial design and analyze the data using analysis of variance or response surface methodology (RSM) (Cui and Zhang, 2012; Supramani et al., 2019; Yu et al., 2022).
Consider that the production of EPS is affected by several variables, such as inoculum properties, time, and culture conditions (Hamidi et al., 2022).
Use a negative control of the culture medium (without inoculum) for later use in the design and for calculating productive parameters.
Carbon sources are among the most crucial variables for EPS production. It is important to utilize at least two different carbon sources when producing EPS, as the production can vary significantly. Glucose or sucrose are proposed in this protocol; however, other conventional and unconventional sources can be used. The concentration of the carbon source varies among different fungal species. The use of 40 g/L is proposed in this protocol; however, other authors reported values up to 120 g/L (Mahapatra and Banerjee, 2013).
During the purification process, filtration is crucial for separating biomass residues. EPS can be used to test cell lines or other biological activity assays.
Calculate the dry EPS, dry biomass, volumetric productivity, specific productivity, practical yield, and recovery efficiency (Fariña et al., 1998; Q. Meng et al., 2021) (Table 2).
Table 2. Fermentation parameters corresponding to the exopolysaccharides (EPS) production using submerged culture
Parameters Equation Dry EPS (g/L) Dry biomass (g/L) Volumetric productivity (VP) Dry EPS (g/L)/Fermentation time (h) Specific productivity VP (g/L/h)/Dry biomass (g/L) Practical yield Dry EPS (g/L/)/Initial carbon source substrate (g/L) Recovery efficiency Lyophilized EPS (g/L)/Dry EPS (g/L) Analyze the data using RStudio or MATLAB. The data correspond to the determination of the equations in Table 2.
Determine the purity of the EPS by quantifying total polysaccharides, phenols, and proteins. The methodologies for these determinations are available in Parimelazhagan (2016).
Validation of protocol
Results for dry EPS are presented in Table 3. The results are reported as mean ± standard deviation, n = 3 for this work.
Table 3. Comparison of dry exopolysaccharides (EPS) corresponding to its production by macrofungi strains using submerged culture
| Macrofungi | Reference | Condition culture | Dry EPS (g/L) |
| Schizophyllum radiatum Fr | This work | pH 5.5, SYAMI medium, 40 g/L of sucrose, 7 days, 150 rpm | 10.58 ± 3.28 |
| Lentinus crinitus (L.) Fr | This work | pH 5.5, SYAMI medium, 7 days, 40 g/L sucrose, 150 rpm | 4.61 ± 1.30 |
| Schizophyllum radiatum Fr | Meng et al., 2021 | pH-, seed medium, 6 days, 40 g/L of glucose, 1.0 g/L of Tween 80 | 3.77 |
| Lentinus tigrinus (Bull.) Fr. | He et al., 2016 | Lactose 60 g/L, yeast extract 4 g/L; pH = 5.0, 12 days, 0.2 % Tween 80. | 4.10 ± 0.27 |
General notes and troubleshooting
The experimental procedures necessary to carry out the protocol involve the use of chemical substances with a low risk of ignition and irritation by contact with the skin, eyes, and mucous membranes, so all protection elements and safety measures must be used to avoid and prevent accidents.
Acknowledgments
This study was supported by Project No. 58653, Alianza Colombia Científica, Gobierno Nacional de Colombia y Banco Mundial, Proyecto Betaglucanes, Contrato FP448442-211-2018 of 2018. This protocol was adapted from the work carried out by Fariña et al. (2001). We thank Dr. Julia Fariña, PROIMI, CABBIO and MINCIENCIAS for the course entitled: “Mycoprospecting: from nature to biotechnological product. Production in bioreactors and downstream processing.”
Competing interests
The authors declare that they have no conflicts of interest.
Ethical considerations
The specimens used in this study were collected under a permit conceded for access to biological resources for non-commercial purposes (Permiso Marco de Recolección, Resolución 2191 de 2018, Universidad del Tolima) and contract acknowledged for access to genetic resources and derivate products for non-commercial purposes (Contrato Marco de Acceso a Recursos Genéticos y sus Productos Derivados No. 294 de 2020. Expediente RGE0353-3, Universidad del Tolima).
References
Angelova, G., Brazkova, M., Mihaylova, D., Slavov, A., Petkova, N., Blazheva, D., Deseva, I., Gotova, I., Dimitrov, Z., Krastanov, A., et al. (2022). Bioactivity of Biomass and Crude Exopolysaccharides Obtained by Controlled Submerged Cultivation of Medicinal Mushroom Trametes versicolor. J. Fungi 8(7): 738.
- Arora, S. and Tandon, S. (2015). Mushroom Extracts Induce Human Colon Cancer Cell (COLO-205) Death by Triggering the Mitochondrial Apoptosis Pathway and Go/G1-Phase Cell Cycle Arrest. Arch. Iran. Med. 18(5): 284–295.
- Bacic, A., Fincher, G. B. and Stone, B. (Eds.). (2009). Chemistry, biochemistry, and biology of 1-3 beta glucans and related polysaccharides. Academic Press.
- Breitenbach, R., Gerrits, R., Dementyeva, P., Knabe, N., Schumacher, J., Feldmann, I., Radnik, J., Ryo, M. and Gorbushina, A. A. (2022). The role of extracellular polymeric substances of fungal biofilms in mineral attachment and weathering. npj Mater. Degrad. 6(1): 42.
- Brito, G. C. B., Lange, L. C., Santos, V. L., Amaral, M. C. S. and Moravia, W. G. (2019). Long-term evaluation of membrane bioreactor inoculated with commercial baker’s yeast treating landfill leachate: pollutant removal, microorganism dynamic and membrane fouling. Water Sci. Technol. 79(2): 398–410.
- Castillo, N. A., Valdez, A. L. and Fariña, J. I. (2015). Microbial production of scleroglucan and downstream processing. Front. Microbiol. 6: e01106.
- Cui, J. D. and Zhang, Y. N. (2012). Evaluation of Metal Ions and Surfactants Effect on Cell Growth and Exopolysaccharide Production in Two-Stage Submerged Culture of Cordyceps militaris. Appl. Biochem. Biotechnol. 168(6): 1394–1404.
- Dudekula, U. T., Doriya, K. and Devarai, S. K. (2020). A critical review on submerged production of mushroom and their bioactive metabolites. 3 Biotech 10(8): e1007/s13205-020-02333-y.
- Fariña, J. I., Sineriz, F., Molina, O. E. and Perotti, N. I. (2001). Isolation and physicochemical characterization of soluble scleroglucan from Sclerotium rolfsii. Rheological properties, molecular weight and conformational characteristics. Carbohydr. Polym. 44(1): 41–50.
- Fariña, J., Siñeriz, F., Molina, O. E. and Perotti, N. I. (1998). High scleroglucan production by Sclerotium rolfsii: Influence of medium composition. Biotechnol. Lett. 20: 825–831.
- Gong, P., Wang, S., Liu, M., Chen, F., Yang, W., Chang, X., Liu, N., Zhao, Y., Wang, J., Chen, X., et al. (2020). Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides: A mini-review. Carbohydr. Res. 494: 108037.
- Hamidi, M., Okoro, O. V., Milan, P. B., Khalili, M. R., Samadian, H., Nie, L. and Shavandi, A. (2022). Fungal exopolysaccharides: Properties, sources, modifications, and biomedical applications. Carbohydr. Polym. 284: 119152.
- Huang, X. and Nie, S. (2015). The structure of mushroom polysaccharides and their beneficial role in health. Food Funct. 6(10): 3205–3217.
- He, P., Wu, S., Pan, L., Sun, S., Mao, D., & Xu, C. (2016). Effect of Tween 80 and acetone on the secretion, structure and antioxidant activities of exopolysaccharides from Lentinus tigrinus. Food Technology and Biotechnology, 54(3), 290-295.
- Isikhuemhen, O. S., Mikiashvili, N. A., Senwo, Z. N. and Ohimain, E. I. (2014). Biodegradation and Sugar Release from Canola Plant Biomass by Selected White Rot Fungi. Adv. Biol. Chem. 4(6): 395–406.
- Jaros, D., Köbsch, J. and Rohm, H. (2018). Exopolysaccharides from Basidiomycota: Formation, isolation and techno-functional properties. Eng. Life Sci. 18(10): 743–752.
- Kambourova, M., Oner, E. T. and Poli, A. (2015). Exopolysaccharides from Prokaryotic Microorganisms—Promising Sources for White Biotechnology Processes. Industrial Biorefineries and White Biotechnology: 523–554.
- Li, N., Zhang, X., Wang, D., Cheng, Y., Wu, L. and Fu, L. (2017). Contribution characteristics of the in situ extracellular polymeric substances (EPS) in Phanerochaete chrysosporium to Pb immobilization. Bioprocess. Biosyst. Eng. 40(10): 1447–1452.
- Mahapatra, S. and Banerjee, D. (2013). Fungal Exopolysaccharide: Production, Composition and Applications. Microbiol. Insights 6: MBI.S10957.
- Meng, Q., Chuai, S., Chen, L., Wang, L., Cai, G., Mao, J., Gu, Z., Shi, G. and Ding, Z. (2021). Effect of surfactants on the production of polysaccharides from Schizophyllum commune through submerged fermentation. Int. J. Biol. Macromol. 192: 210–218.
- Meng, X., Liang, H. and Luo, L. (2016). Antitumor polysaccharides from mushrooms: a review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 424: 30–41.
- Nowakowski, P., Markiewicz-Żukowska, R., Bielecka, J., Mielcarek, K., Grabia, M. and Socha, K. (2021). Treasures from the forest: Evaluation of mushroom extracts as anti-cancer agents. Biomed. Pharmacother. 143: 112106.
- Papagianni, M. (2004). Fungal morphology and metabolite production in submerged mycelial processes. Biotechnology advances. 22(3), 189-259.
- Parimelazhagan, T. (2016). Pharmacological assays of plant-based natural products. Springer.
- Seneviratne, G., Zavahir, J. S., Bandara, W. M. M. S. and Weerasekara, M. L. M. A. W. (2008). Fungal-bacterial biofilms: their development for novel biotechnological applications. World J. Microbiol. Biotechnol. 24(6): 739–743.
- Supramani, S., Ahmad, R., Ilham, Z., Suffian Mohamad Annuar, M., Klaus, A. and Abd Al Qadr Imad Wan-Mohtar, W. (2019). Optimisation of biomass, exopolysaccharide and intracellular polysaccharide production from the mycelium of an identified Ganoderma lucidum strain QRS 5120 using response surface methodology. AIMS Microbiol. 5(1): 19–38.
- Wang, W., Tan, J., Nima, L., Sang, Y., Cai, X. and Xue, H. (2022). Polysaccharides from fungi: A review on their extraction, purification, structural features, and biological activities. Food Chem.: X 15: 100414.
- Yu, C., Fang, Y., Huang, W., Lei, P., Xu, X., Sun, D., Wu, L., Xu, H. and Li, S. (2022). Effect of surfactants on the production and biofunction of Tremella fuciformis polysaccharide through submerged fermentation. LWT 163: 113602.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Dávila Giraldo, L. R., Villanueva Baez, P. X., Zambrano Forero, C. J. and Arango, W. M. (2023). Production, Extraction, and Solubilization of Exopolysaccharides Using Submerged Cultures of Agaricomycetes. Bio-protocol 13(19): e4841. DOI: 10.21769/BioProtoc.4841.
Category
Biological Sciences > Biological techniques
Microbiology > Microbial biochemistry > Carbohydrate
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








