- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Rapid and Simple Procedure for the Isolation of Embryonic Cells from Fish Eggs
Published: Vol 13, Iss 19, Oct 5, 2023 DOI: 10.21769/BioProtoc.4836 Views: 1359
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
A Cold-Active Protease Tissue Dissociation Protocol for the Preservation of the Tendon Fibroblast Transcriptome
Arul Subramanian [...] Thomas F. Schilling
May 5, 2025 2676 Views
Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1260 Views
Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1148 Views
Abstract
Fertilized teleost fish eggs are a complex formation, in which dividing cells arelocated in a small point in the entire volume of eggs. Isolating embryonic cellscan be considered a necessary step in the research of developmentalpeculiarities of fish cells at the earliest stages of embryogenesis beforeembryo formation. The main advantages of the offered protocol are rapidisolation, no enzymes, and overall low cost compared to other protocols. Theprotocol is suitable for the isolation of embryonic cells from medium-sized eggsat the stages of blastula or gastrula, for studies in a variety of applications(e.g., microscopy, flow cytometry, and other methods). Fertilized nelma eggs(Stenodus leucichthys nelma) are used in the protocol as a model.
Key features
• Fast and cheap isolation of cells from fish eggs at early stages (blastula orgastrula).
• Applicable for most of the methods for cell study (any staining, microscopy, flowcytometry, etc.).
• Can be applied to other teleost fish eggs with medium egg diameter of 3–4mm.
Graphical overview

Background
To date, many methods for isolating living cells from various tissues are used fordifferent purposes. Mainly, living cells from tissues are disaggregated bytrypsinization. This method is very common and involves the extraction of an embryoor tissue with further trypsinization (Durkin et al.,2013). Some other protocols are based on mechanical crushing of tissues orformed embryos to obtain cells (Fetherman et al., 2015).
When rapid testing of samples is required as soon as possible after eggfertilization, it is necessary to isolate cells directly from the blastodisc at theearliest stages of teleost fish embryogenesis before formation of embryo, larva, orfry. Cell isolation from fertilized eggs by the methods described above does notlead to the desired result, since the eggs contain huge amounts of varioussubstances and a small number of cells relative to the weight of the egg. Inaddition, the cells in the blastodisc are not tightly connected with each other.That is why the use of trypsin (and other enzymes) is not ideal, with undesirablechemical effects on the cells that can lead to the destruction of cell membranes.
To date, we have only found one other protocol describing a similar procedure (Rieger, 2019). That protocol requires thepresence of pronase from Streptomyces griseus for thedechorionization of embryos. The pronase only softens the chondrion, requiringadditional washing to remove it from the embryo. Besides, the isolation procedure iscomplicated by particular features of enzymes, as most biological catalysts havenarrow operating limits as well as a short shelf life. We aimed to ensure maximumsimplicity and low cost of the isolation method with minimal requirements forlaboratory equipment.
Materials and reagents
Biological materials
Fertilized fish eggs in the stages from blastodisc to the late gastrula.
Solutions
PBS soluble tablets (Sigma-Aldrich, catalog number: P4417), for cell isolation
70% ethanol solution (Sigma-Aldrich, catalog number: 65348-85), for cell preservation
o-safranin ready-to-use solution 0.1% (Scientific Laboratory Supplies, catalog number: TMS-009-C), for protocol verification
Propidium iodide ready-to-use solution 1 mg/mL (Thermo Fisher Scientific, catalog number: P3566), for protocol verification
RNAse A ready-to-use solution 10 mg/mL (Thermo Fisher Scientific, catalog number: EN0531)
Phosphate-saline buffer solution (see Recipes)
70% ethanol (see Recipes)
Recipes
Phosphate-saline buffer solution
Reagent Final concentration Quantity PBS 150 mM total of all salts:
137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 2 mM KH2PO4
1 tablet H2O n/a Up to 100 mL Total n/a 100 mL 70% ethanol
Reagent Final concentration Quantity Ethanol (95%) 70% 74 mL H2O n/a 26 mL Total n/a 100 mL
Laboratory supplies
Microcentrifuge tube pestles (Sigma-Aldrich, catalog number: BAF199230001-100EA)
Conical bottom microcentrifuge tubes (Sigma-Aldrich, catalog number: HS4325-1000EA)
Centrifuge tubes (Sigma-Aldrich, catalog number: CLS430790)
Equipment
Microcentrifuge (Thermo Fisher Scientific, catalog number: 75004081)
Microscope with 8–20× zoom lens for data validation (Thermo Fisher Scientific, catalog number: 4479672)
Vortex mixer (VELP Scientifica, catalog number: F202A0173)
Dissecting needles (Thermo Fisher Scientific, catalog number: 13-820-024)
Flow cytometer for data validation (Beckman Coulter, catalog number: CO9752)
Software and datasets
CytExpert software for flow cytometry (used for data validation)
Procedure
This protocol has been tested on fertilized nelma eggs of medium size (3–4 mm in diameter) among teleost fish (including zebrafish). Before the procedure, rinse the eggs with PBS: place the eggs in a centrifuge tube (15 mL) and pour 10–20 mL of cool PBS solution into it. Drain the liquid and repeat this step to prevent any contamination that could affect further analysis. This step can also be done in any way available in the laboratory: using a tea strainer, beakers, gauze, etc.
Using a pestle, pop the eggs (or single egg) in the tube. It is better to add eggs and process them gradually. Do not homogenize nor strongly push the pestle to avoid damage to the cells: it is enough to break the shell. The sample volume from the processed eggs should not exceed 2/3 of the tube. A 1.5 mL centrifuge tube can be fitted with a different number of eggs, depending on their size. Thus, approximately 30 eggs of 3–4 mm diameter can be sequentially placed in the tube during the process.
Fill the tube with eggs with cold PBS and shake it on a vortex for 5 s or mechanically using a pestle or dissecting needle to release the contents of all eggs outside the visible shell.
Mechanically remove all shells from the tube using a dissecting needle. It is very important to ensure that there are no residual shells in the resulting translucent medium to avoid cell loss.
Centrifuge at 300–500× g for 5 min at 4 °C.
Discard the supernatant without affecting the precipitate. Add 1–1.5 mL of cold PBS and centrifuge again.
(Optional) Repeat step 6 1–2 times to wash the cells out of small debris and resuspend the pellet in 100 μL of cold PBS.
Following these steps, we obtain a pellet containing the cells. The volume of PBS can be changed as desired (50–500 μL). This suspension contains a certain amount of small suspended non-cellular particles. After the procedure, the total proportion of cells among other floating non-cellular particles in the suspension is at least 10%.
The cell suspension is ready to use for any analysis. It can also be fixed for long-term cell storage using standard protocols such as using chilled ethanol (Ciancio et al., 1988).
Data analysis
Flow cytometry and microscopy were used for protocol verification. The CytExpert software was used for all cytometry data.
Validation of protocol
Maturating nelma eggs (Stenodus leucichthys nelma) were used to validate the protocol (Figure 1A).
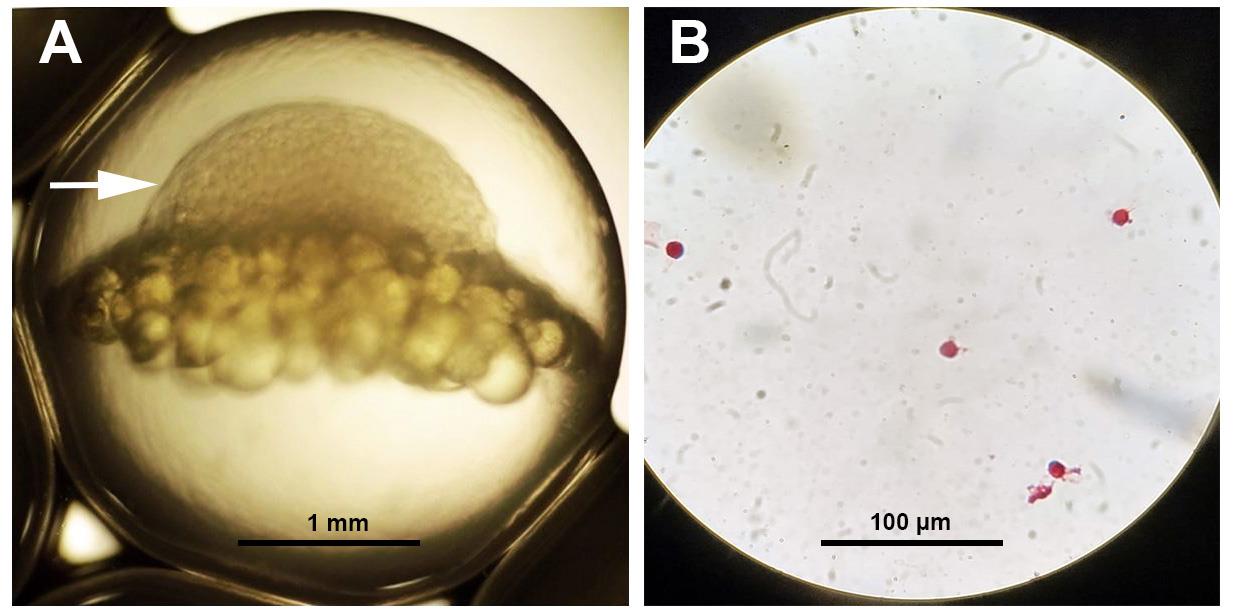
Figure 1. Maturating Stenodus leucichthys nelma egg. (A) White arrow shows the position of the blastodisc. (B) Isolated cells from the blastodisc stained with O-safranin.
Thirty fish eggs were processed by the protocol described above. Then, the cell-containing pellet was resuspended in 300 μL of PBS and fixated in 70% ethanol (Ciancio et al., 1988) to ensure long-term preservation prior to analysis. A small 10 μL aliquot of the obtained suspension was stained with safranin (Swain and De, 1990). The suspension was air-dried at room temperature on a glass slide causing the cells to adhere to it. Then, the slide was immersed in a solution of safranin for 5 min and washed one time with PBS. The result was checked using a light microscope (Figure 1B ).
In order to verify the results and additionally show the real presence of isolated cells, an alcohol suspension of the obtained cells (300 μL) was stained with propidium iodide (Riccardi and Nicoletti, 2006). The alcohol was washed off the cells twice with PBS by centrifugation at 300× g for 5 min; cells were then resuspended in 100 μL of PBS (+0.1 μg/mL RNase A and 0.01 μg/mL propidium iodide). After 30 min of incubation in the dark at 4 °C, stained cells were analyzed using a flow cytometer (channel PE) (Figure 2). The flow cytometer analyzed 500,000 events. Using a software interface, side scatter, and forward scatter data, only single cells were selected for analysis. The histogram along the PE channel clearly showed peaks corresponding to the cell cycle of isolated cells (Figure 2A). The vertical line on the dot plot (Figure 2B) showed the position corresponding to the G1 peak of the cell cycle in the histogram (Figure 2A). On the dot plot diagram (Figure 2B) for the same channel, there is size cell dispersion (y-axis) that corresponds to the stage of embryogenesis (small cell blastula) where cells acquire different sizes (Figure 2B). According to our data, approximately 100,000 cells were isolated (Figure 2B) from 30 eggs (Figure 1A), which was quite commensurate with other known methods.
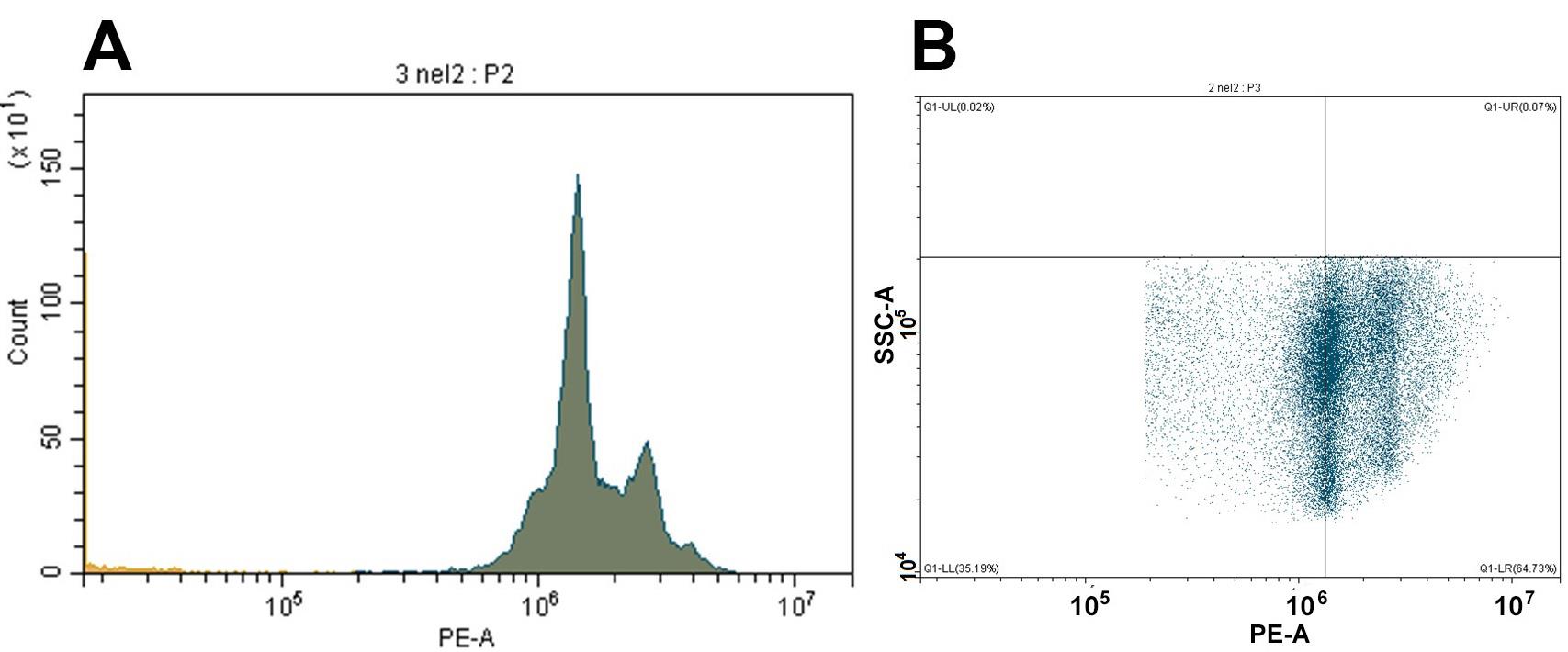
Figure 2. Results of flow cytofluorometry (PE channel). Cell cycle histogram (A) and dot plot (B) showing the heterogeneity of the cell analyzed from small cell blastula.
Thus, the protocol was tested in two independent ways: visual (microscopic) and photometric. The procedure efficiency for cell isolation from fish egg blastodiscs was proved. The protocol can be applied to most teleost fish species with middle-sized eggs at early maturating stages (blastula, gastrula); this protocol may be suitable for other representatives of teleost fish with different diameters of eggs [large (salmon) or small (zebrafish)].
General notes and troubleshooting
General notes
Before you start: to isolate cells from the blastodisc, it is enough to have developing eggs at the stage of large cell blastula. Based on specific goals, it is important to mind the number of cells needed for analysis. If it is necessary to analyze each egg individually, it is important to know the total number of required cells in the developing egg. For examining a specific number of eggs (if general statistics on the target analysis is needed), the stage of development of the eggs is not so important.
Troubleshooting
No cells after procedure:
Take more eggs for processing and repeat the procedure.
Ensure that you have taken the developing eggs. An embryo should be visible under a light microscope.
Remember that in the first step of the isolation protocol, after rinsing the eggs, it is sufficient to gently pop the eggs. Do not completely homogenize.
Acknowledgments
This study was supported by the RSF grant 23-26-00257.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Ciancio, G., Pollack, A., Taupier, M. A., Block, N. L. and Irvin, G. L. (1988). Measurement of cell-cycle phase-specific cell death using Hoechst 33342 and propidium iodide: preservation by ethanol fixation. J. Histochem. Cytochem. 36(9): 1147–1152.
- Durkin, M., Qian, X., Popescu, N. and Lowy, D. (2013). Isolation of Mouse Embryo Fibroblasts. Bio Protoc 3(18): e908.
- Fetherman, E. R., Lepak, J. M., Brown, B. L. and Harris, D. J. (2015). Optimizing Time of Initiation for Triploid Walleye Production Using Pressure Shock Treatment. North Am. J. Aquacult. 77(4): 471–477.
- Riccardi, C. and Nicoletti, I. (2006). Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 1(3): 1458–1461.
- Rieger, S. (2019). Dechorionation of zebrafish embryos with Pronase for metronidazole-mediated ß-cell ablation V.2. DiaComp Protocols.
- Swain, D. and De, D. N. (1990). Differential Staining of the Cell Cycle of Plant Cells Using Safranin and Indigo-Picrocarmine. Stain Technol. 65(4): 197–204.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Golotin, V., Lyutikov, A., Filatova, T., Sharoyko, V. and Apalikova, O. (2023). A Rapid and Simple Procedure for the Isolation of Embryonic Cells from Fish Eggs. Bio-protocol 13(19): e4836. DOI: 10.21769/BioProtoc.4836.
Category
Developmental Biology
Cell Biology > Cell isolation and culture > Cell isolation
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








