- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Production and Purification of Cell Culture–generated Hepatitis B Virus by Transient Transfection and Density Gradient
Published: Vol 13, Iss 14, Jul 20, 2023 DOI: 10.21769/BioProtoc.4779 Views: 1897
Reviewed by: Saskia F. ErttmannAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Flotation Assay With Fluorescence Readout to Study Membrane Association of the Enteroviral Peripheral Membrane Protein 2C
Kasturika Shankar [...] Lars-Anders Carlson
Apr 5, 2025 1604 Views

Detection and Analysis of S-Acylated Proteins via Acyl Resin–Assisted Capture (Acyl-RAC)
Dina A. Abdulrahman and Michael Veit
Apr 5, 2025 1826 Views
Inducible HIV-1 Reservoir Reduction Assay (HIVRRA), a Fast and Sensitive Assay to Test Cytotoxicity and Potency of Cure Strategies to Reduce the Replication-Competent HIV-1 Reservoir in Ex Vivo PBMCs
Jade Jansen [...] Neeltje A. Kootstra
Jul 20, 2025 2457 Views
Abstract
An efficient cell culture system for hepatitis B virus (HBV) is indispensable for research on viral characteristics and antiviral agents. Currently, for HBV infection assays in cell culture, HBV genome-integrated cell line–derived viruses are commonly used. However, these viruses are not suitable for the evaluation of polymorphism-dependent viral characteristics or resistant mutations against anti-viral agents. To detect the infection of cell culture–generated HBV (HBVcc) by the transient transfection of the HBV molecular clone, a large amount of purified viruses is needed, because such viruses exhibit limited infection efficiencies in cell culture. Here, we describe how to generate and purify HBVcc by the transient transfection of HBV molecular clones. This system provides a powerful tool for studying the infection and propagation of HBV and for developing anti-viral agents against HBV.
Keywords: HBVBackground
Hepatitis B virus (HBV) infection is a significant cause of chronic liver disease, including cirrhosis and hepatocellular carcinoma. Although effective vaccines against HBV infection are available in many countries, the global prevalence of HBV is estimated to be over 290 million. The eradication of chronic HBV infection by the current treatment strategy is not expected, because HBV covalently closed circular DNA in hepatocytes cannot be eliminated. To explore novel anti-HBV agents, a system for the infection and replication of HBV in cell culture is indispensable. Sodium taurocholate cotransporting polypeptide (NTCP) was identified as an HBV receptor, and NTCP-transduced HepG2 or HuH-7 cells contributed to the observation of HBV infection and replication in cell culture (König et al., 2019; Otoguro et al., 2020). In such cell culture systems for HBV, viruses derived from HBV genotype D genome-integrated cell lines, such as HepG2.2.15 or HepAD-38, are used. However, such viruses are not suitable for investigating the effects of strain-specific characteristics or resistance-related polymorphisms on the effectiveness of anti-HBV agents. For these purposes, the viruses obtained by the transient transfection of the HBV molecular clone are used. However, these viruses have limited infection efficiencies in cell culture. Therefore, methods for the efficient production of cell culture–generated HBV (HBVcc) and for the purification of infectious viruses will be important (Washizaki et al., 2022).
Materials and reagents
Production of HBVcc
HBV molecular clone plasmid
A replication-competent HBV molecular clone with a 1.38-fold genome length of HBV genotype C strain (accession number: AB246345) (Figure 1) (Murayama et al., 2021) inserted into the pUC19 plasmid is prepared. This clone is introduced with a precore stop mutation (G1896A) to evaluate the production of HBc proteins by measuring the HBcrAg level excluding HBeAg. HBeAg is not produced from this construct.
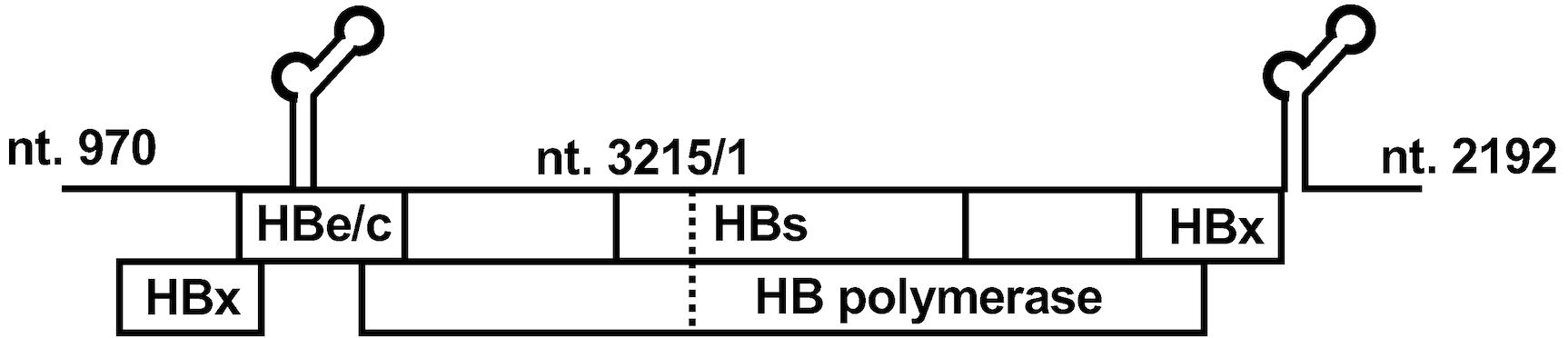
Figure 1. Structure of the hepatitis B virus (HBV) molecular clone for cell culture-generated HBV (HBVcc). Structure of the plasmid of the HBV molecular clone. This plasmid contains a 1.38-fold HBV genome (4,438 bp) of the HBV genotype C strain. This construct generates pregenomic RNA and expresses all HBV proteins.Sodium taurocholate cotransporting polypeptide (NTCP)-transduced cells
HepG2-NTCPsec+ (provided by Dr. Marc Peter Windisch; Institut Pasteur Korea, Seoul, South Korea) (König et al., 2019). These cells were cultured with HepG2-NTCPsec+ culture medium (see Recipes) supplemented with Blasticidin (5µ g/mL)
Dulbecco’s modified Eagle medium (DMEM) (FUJIFILM Wako Pure Chemical Corporation, catalog number: 044-29765)
Fetal bovine serum (FBS) (Sigma-Aldrich, catalog number: F7524)
MEM non-essential amino acids solution (100×) (NEAA) (Thermo Fisher Scientific, Gibco, catalog number: 11140050)
HEPES (1 M) (Thermo Fisher Scientific, Gibco, catalog number: 15630130)
Sodium pyruvate (100 mM) (Thermo Fisher Scientific, Gibco, catalog number: 11360070)
Penicillin-streptomycin (10,000 U/mL) (Thermo Fisher Scientific, Gibco, catalog number: 15140122)
Blasticidin S hydrochloride, HEPES solution (Blasticidin) (10 mg/mL) (FUJIFILM Wako Pure Chemical Corporation, catalog number: 022-18713)
Opti-MEM I reduced serum medium (Thermo Fisher Scientific, Gibco, catalog number: 31985070)
Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific, Invitrogen, catalog number: L3000015)
Collagen-coated T225 flask (Corning, catalog number: NCO431082)
Dulbecco’s phosphate buffered saline (PBS) (Nacalai Tesque, catalog number: 14249-24)
Dimethyl sulfoxide (DMSO) (Nacalai Tesque, catalog number: 09659-14)
Syringe-top filter unit; Millex-HV Syringe Filter Unit, 0.45 μm, PVDF, 33 mm (Merck Millipore, catalog number: SLHVR33RS)
Amicon Ultra-15 centrifugal filter units (100 kDa) (Merck Millipore, catalog number: UFC910096)
Purification of HBVcc
Optiprep (60% iodixanol solution) (Serumwerk Bernburg, catalog number: AXS-1114542)
Open-top thinwall ultra-clear tube (14 mm × 89 mm) (Beckman Coulter, catalog number: 344059)
DNase; RQ1 RNase-free DNase (Promega, catalog number: M6101)
QIAamp DNA Mini kit (Qiagen, catalog number: 51306)
Lumipulse G HBsAg-Quant (Fujirebio, catalog number: 296851)
Lumipulse G HBcrAg (Fujirebio, catalog number: 294109)
Luna Universal qPCR Master Mix (New England Biolabs, catalog number: M3003)
Primers and probe for the real-time PCR targeting the HBs region:
Forward primer: 5′-CTTCATCCTGCTGCTATGCCT-3′
Reverse primer: 5′-AAAGCCCAGGATGATGGGAT-3′
Probe: 5′-FAM-ATGTTGCCCGTTTGTCCTCTAATTCCA-TAMRA-3′
TNE buffer (see Recipes)
Titration of HBVcc
Collagen-coated 96-well culture plate (Corning, catalog number: NCO3585)
Polyethylene glycol average mol wt 8,000 (PEG8000) (Sigma-Aldrich, catalog number: P2139)
4% paraformaldehyde phosphate buffer solution (4% PFA) (FUJIFILM Wako Pure Chemical Corporation, catalog number: 163-20145)
Block ACE (Bio-Rad, catalog number: BUF029)
Triton X-100 detergent (Calbiochem, Merck Millipore, catalog number: 648466)
Anti-HBc antibody; anti-hepatitis B virus core antigen IgG fraction (polyclonal) (AUSTRAL Biologicals, catalog number: HBP-023-9)
Goat anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor Plus 555 (Thermo Fisher Scientific, catalog number: A327320
DAPI (4’,6-Diamidino-2-phenylindole dihydrochloride) (FUJIFILM Wako Pure Chemical Corporation, catalog number: 342-07431)
4% PEG8000 (see Recipes)
Recipes
HepG2-NTCPsec+ culture medium
DMEM
10% FBS
Penicillin-streptomycin (100 U/mL)
NEAA (1×)
HEPES (10 mM)
Sodium pyruvate (1 mM)
TNE buffer
150 mM NaCl
10 mM Tris HCl (pH 7.5)
1 mM EDTA
4% PEG8000
Dissolve in water to 4% (w/v) in a water bath at 60 °C.
Equipment
Refrigerated centrifuge with swing rotor (TOMY, model: AX-310)
Automated chemiluminescent enzyme immunoassay system (Fujirebio, model: LUMIPULSE G1200)
Ultracentrifuge apparatus, Optima L-90K with an SW-41 Ti rotor (Beckman Coulter, model: Optima L-90K)
Analytical balance (Sartorius Lab Instruments, model: SECURA124-1SJP)
Real-time PCR system (Thermo Fisher Scientific, model: StepOnePlus Real-Time PCR System)
Fluorescence microscope (KEYENCE, model: BZ-X710)
Procedure
Production of HBVcc
Detach and count HepG2-NTCPsec+ cells.
Place the cells (5 × 107 cells resuspended with 2–5 mL HepG2-NTCPsec+ culture medium) into a 50 mL conical tube.
Prepare the transfection mixture in 50 mL conical tubes (Table 1).
Table 1. Composition of transfection reagent
Reagent Amount Mixture A Opti-MEM I reduced serum medium 2 mL HBV molecular clone plasmid (2 μg/μL) 40 μL P3000 enhancer reagent (included in Lipofectamine 3000 Transfection Reagent kit) 80 μL Mixture B Opti-MEM I reduced serum medium 2 mL Lipofectamine 3000 reagent 80 μL Mix the prepared mixtures A and B and incubate for 5 min following the manufacturer’s protocol.
Transfer the generated mixture into HepG2-NTCPsec+ cells in the 50 mL conical tube and mix well by vortex.
Seed the transfected cells into a collagen-coated T225 flask with 45 mL of HepG2-NTCPsec+ culture medium.
One day after transfection, wash the transfected and seeded cells with 12 mL of PBS three times and add 45 mL of HepG2-NTCPsec+ culture medium with 2% DMSO. The addition of DMSO improves the yield of HBVcc.
Culture the transfected cells for an additional seven days, monitoring HBsAg levels in the culture medium by the automated chemiluminescent enzyme immunoassay system (Murayama et al., 2019) (Figure 2).
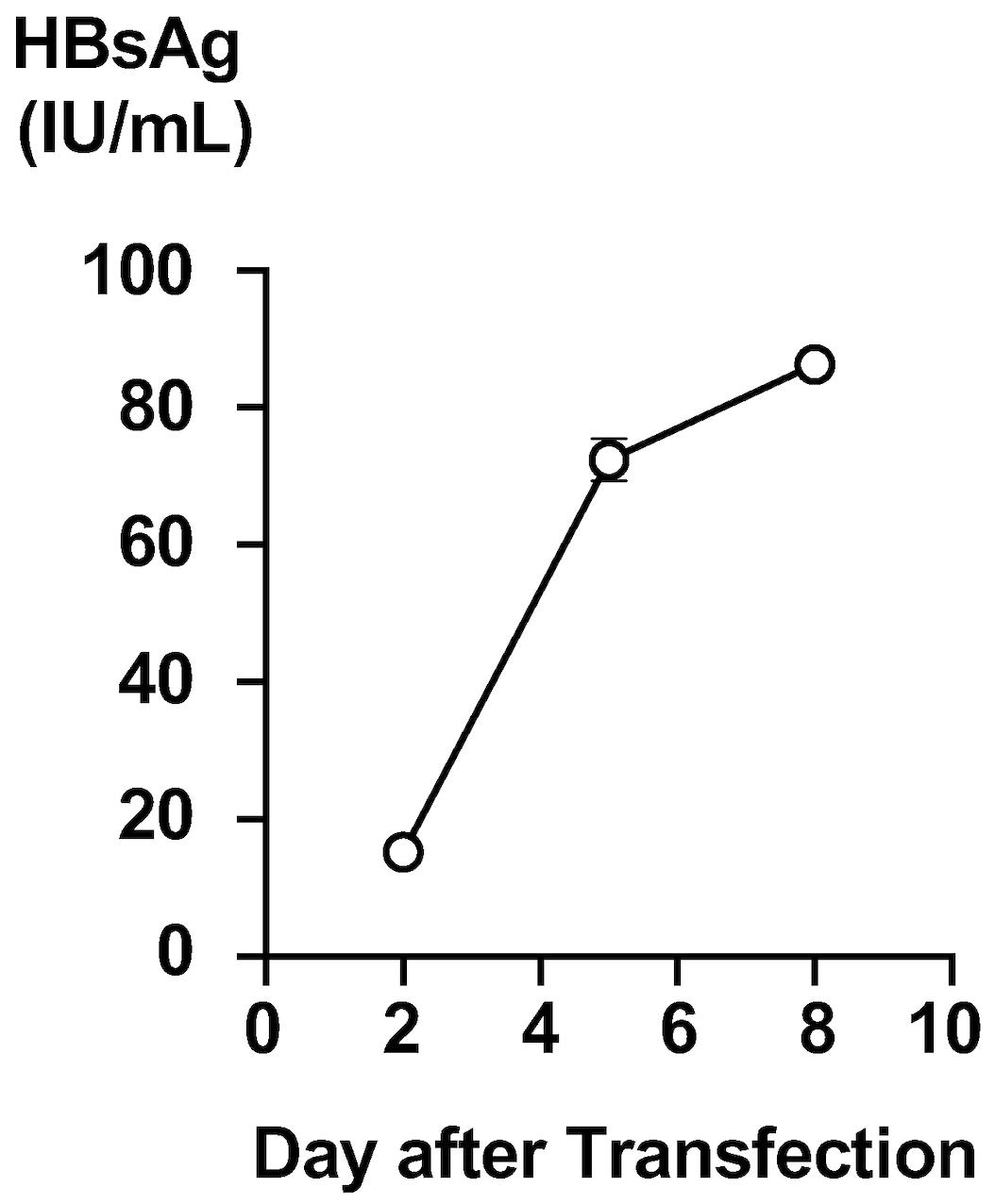
Figure 2. Production of HBsAg in the culture medium of hepatitis B virus (HBV) molecular clone-transfected cells. HBsAg levels in the culture medium of the HBV molecular clone-transfected cells were monitored. A time-dependent increase was observed.Eight days after transfection, harvest the culture medium into a 50 mL conical tube and centrifuge (2,380× g, 5 min, 4 °C) to precipitate the cell debris.
Pass the culture medium through a 0.45 μm syringe-top filter unit to remove cell debris.
Concentrate the culture medium approximately 30-fold by centrifugation (2,380× g, 90 min, 4 °C) with Amicon Ultra-15 centrifugal filter units (100 kDa) (Table 2). If the recovery rate is quite low, confirm that the centrifugal filter unit is intact, and the condition of the centrifugation is appropriate. The generated HBVcc in the culture medium can be stored at -80 °C before or after concentration.
Table 2. Concentration of HBVcc in culture medium by using two centrifugal filter units
Before After Volume 30 mL 1 mL HBsAg 86.3 IU/mL 2,190 IU/mL HBsAg recovery rate - 84.6% HBV DNA 4.1 × 109 copies/mL 7.2 × 1010 copies/mL HBV DNA recovery rate - 58.5%
Purification of HBVcc
Dilute 60% iodixanol (Optiprep) to 40%, 30%, 20%, and 10% with TNE buffer (Table 3).
Table 3. Composition of mixtures of iodixanol and TNE buffer
Iodixanol concentration Optiprep TNE buffer Total volume 40% 4 mL 2 mL 6 mL 30% 3 mL 3 mL 6 mL 20% 2 mL 4 mL 6 mL 10% 1 mL 5 mL 6 mL Prepare a stepwise iodixanol density gradient by layering the 40%–10% iodixanol solutions (2 mL each) in the ultracentrifuge tube (open-top thinwall ultra-clear tube) (Figure 3).
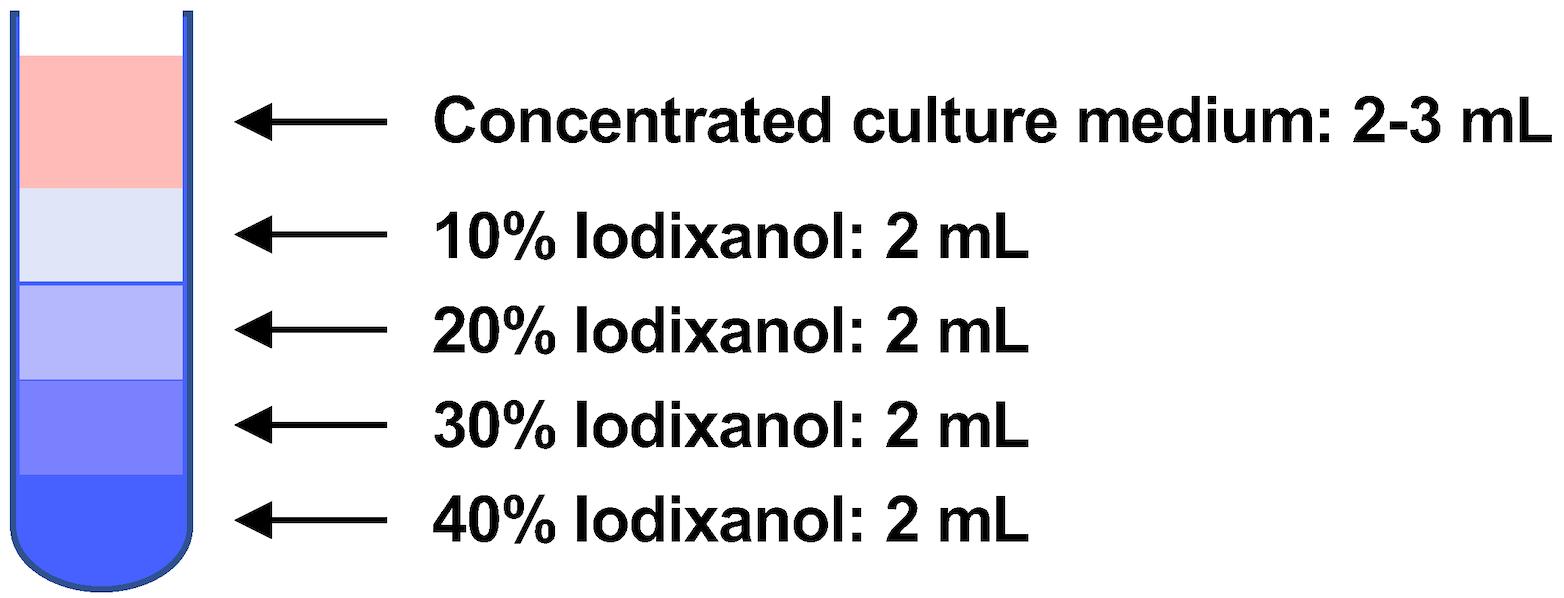
Figure 3. Preparation of stepwise iodixanol density gradient. Two milliliters each of the 40%–10% iodixanol solutions were layered. After that, the concentrated culture medium was put at the top of the iodixanol density gradient.Layer the concentrated culture medium (2–3 mL) on top of the 10%–40% iodixanol gradient.
Centrifuge the culture medium on the gradient at 178,000× g for 16 h at 4 °C in an ultracentrifuge apparatus with an SW-41 Ti rotor.
After centrifugation, collect 500 μL of 20–22 fractions, depending on the volume of the layered concentrated culture medium, from the top of the density gradient. The collected HBVcc in the fractions can be stored at -80 °C.
Take a 100 μL aliquot of each fraction and measure the weight of the 100 μL aliquot using the analytical balance to determine the density of each fraction.
Dilute the collected fraction 200-fold with HepG2-NTCPsec+ culture medium and measure the HBsAg and HBcrAg levels for each fraction by the automated chemiluminescent enzyme immunoassay system as described in the manufacturer’s protocol (Figure 4).
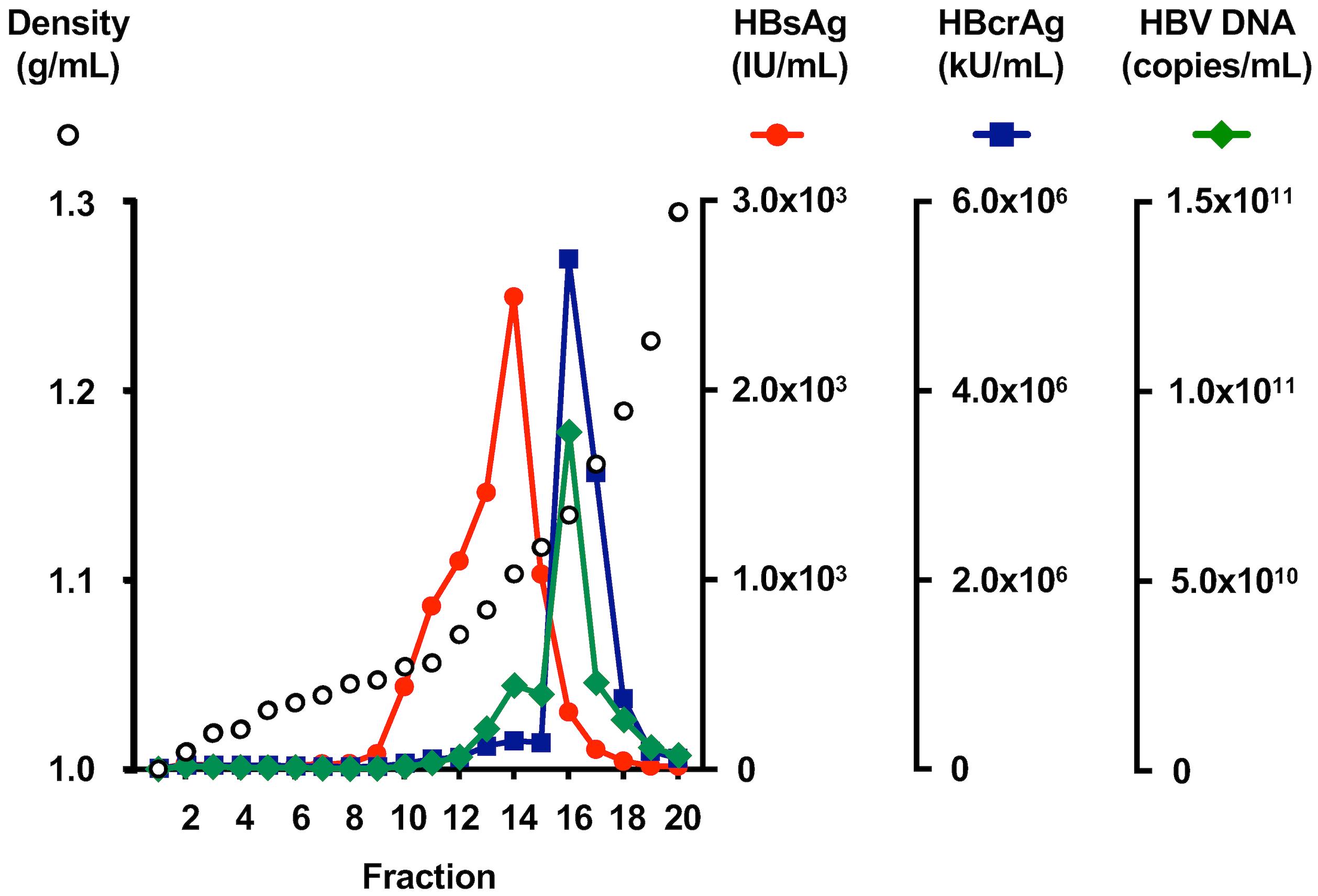
Figure 4. Profile of cell culture–generated hepatitis B virus (HBVcc) in an iodixanol density gradient. HBVcc in the culture medium was applied to an iodixanol density gradient, the titers of HBsAg and HBcrAg were measured in each fraction, and the HBV DNA titer was quantified by real-time PCR.Treat the 50 μL of diluted fraction with DNase (RQ1 RNase-free DNase) at a concentration of 50 unit/mL, 37 °C, 1 h. Extract the total DNA using a QIAamp DNA Mini kit following the manufacturer’s protocol.
Measure the HBV DNA levels by real-time PCR targeting the HBs region by using the plasmid containing the PCR fragment amplified by primers for the real-time PCR as the standard (Tables 4 and 5) (Figure 4) (Honda et al., 2021).
Table 4. Composition of real-time PCR
Reagent Concentration Volume Luna Universal qPCR Master Mix 2× 12.5 μL Forward primer 10 μM 0.5 μL Reverse primer 10 μM 0.5 μL Probe 15 μM 0.5 μL H2O - 6 μL Extracted DNA - 5 μL Table 5. Program for real-time PCR
Step Temperature Time Data collection 1 50 °C 2 min off 2 95 °C 10 min off 3 95 °C 20 s off 4 60 °C 1 min on Repeat steps 3 and 4 for 40 cycles
Titration of HBVcc
Seed HepG2-NTCPsec+ cells in collagen-coated 96-well culture plates at a density of 3 × 104 cells/well in 100 μL of HepG2-NTCPsec+ culture medium supplemented with DMSO (2%).
Prepare the inoculum with an aliquot of each fraction (Table 6) and infect into HepG2-NTCPsec+ cells one day after seeding by replacing the culture medium with the inoculum.
Table 6. Composition of inoculum with purified HBVcc
Amount (per well) Fraction 5 μL HepG2-NTCPsec+ culture medium 39 μL DMSO 1 μL 40% PEG8000 5 μL One day after infection, wash the infected cells with 100 μL of PBS three times and add 100 μL of HepG2-NTCPsec+ culture medium containing 2% DMSO.
Culture the infected cells by changing the culture medium containing 2% DMSO every three or four days.
Collect culture medium 12 days after infection and measure the HBsAg level as described in step A7 (Figure 5A). In this case, fraction 15 was the fraction with the peak infectivity.
After washing with 100 μL of PBS twice, fix the infected cells 12 days after infection by replacing the culture medium with 4% paraformaldehyde in PBS (4% PFA) for 30 min at room temperature (RT).
After washing with 100 μL of PBS twice, block and permeabilize the fixed cells using Block ACE with 0.3% Triton X-100 for 1 h at RT.
Stain the infected cells with anti-HBc antibody (1 μg/mL diluted with PBS) for 1 h at RT.
After washing with 100 μL of PBS three times, stain the infected cells with Alexa Fluor 555-conjugated anti-rabbit IgG (1 μg/mL diluted with PBS) for 1 h at RT in the dark.
After washing with 100 μL of PBS three times, stain the nuclei with DAPI (1 μg/mL diluted with PBS) (Figure 5B).
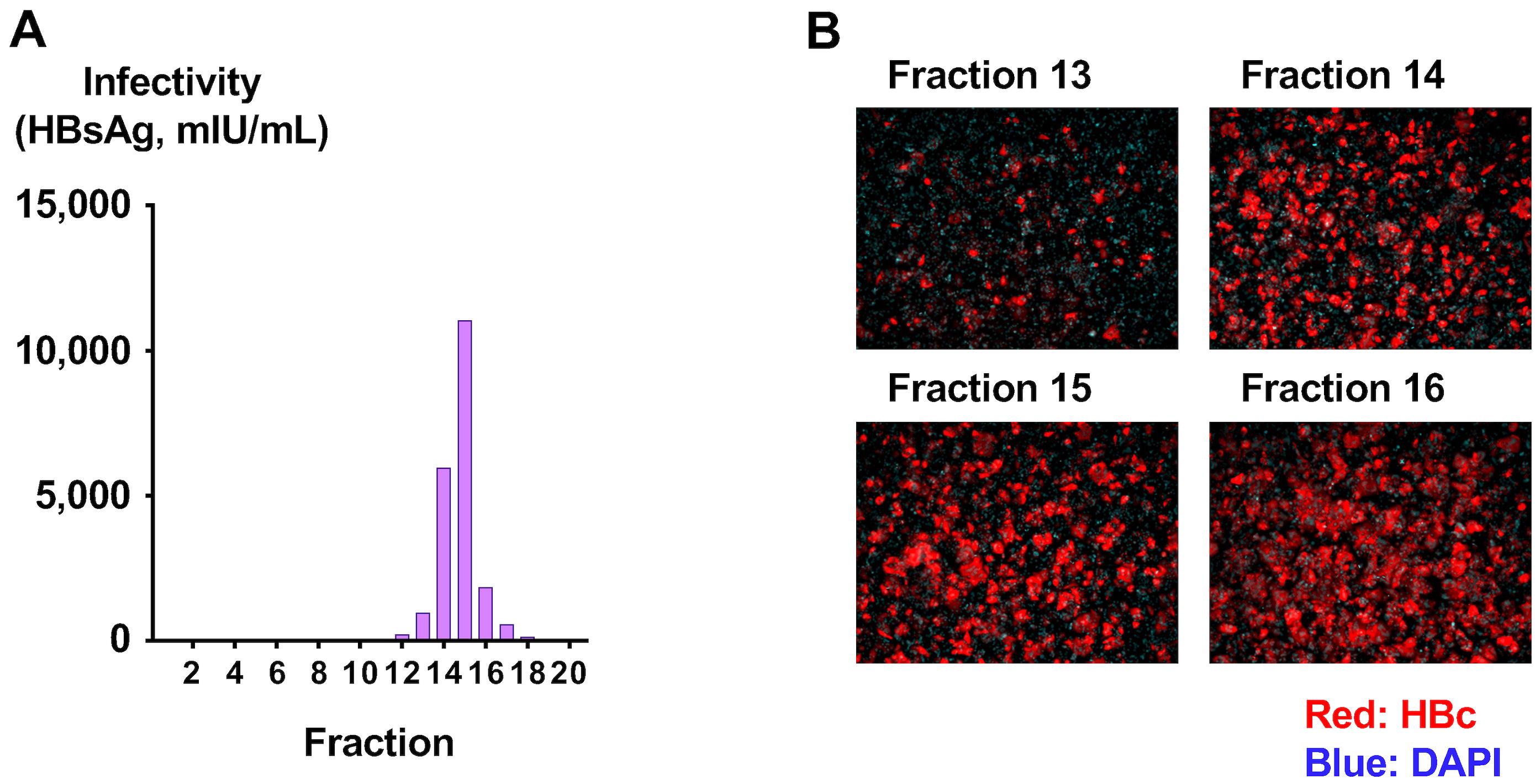
Figure 5. Determination of infectivity of purified cell culture–generated hepatitis B virus (HBVcc). (A) Purified HBVcc was used to infect HepG2-NTCPsec+ cells, and the HBsAg levels in the culture medium at 12 days after infection were measured. (B) The infected cells were fixed and visualized with anti-HBc antibody, and nuclei were visualized by DAPI.To confirm the infectivity of generated viruses in the peak fraction, infect the HBVcc in the peak fraction of infectivity (fraction 15) at concentrations of one genome equivalent (GEq)/cell (3 × 104 copies/well), 10 GEq/cell (3 × 105 copies/well), 100 GEq/cell (3 × 106 copies/well), and 1,000 GEq/cell (3 × 107 copies/well), following the inoculum composition (Table 6).
Measure the HBsAg levels in the culture medium of the infected cells and stain the infected cells with anti-HBc antibody 12 days after infection (see steps A7 and C6–C10) (Figure 6).
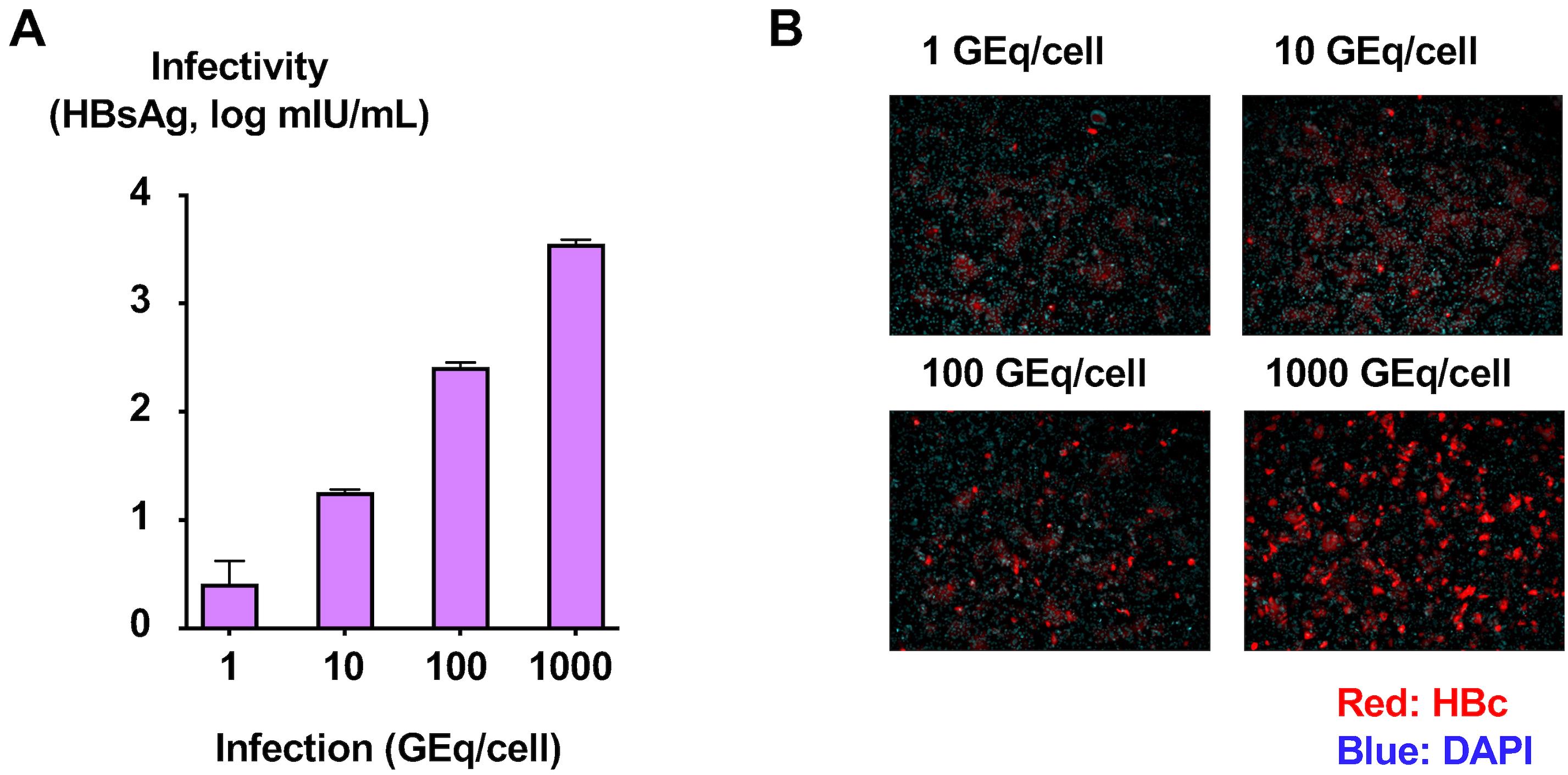
Figure 6. Determination of the infectivity titer of purified cell culture–generated hepatitis B virus (HBVcc). (A) HBVcc in the peak fraction of infectivity was used to infect HepG2-NTCPsec+ cells at concentrations of 1, 10, 100, and 1,000 genome equivalent (GEq)/cell. The HBsAg level in the culture medium at 12 days after infection was measured. (B) The infected cells were fixed and visualized with anti-HBc antibody, and nuclei were visualized by DAPI.
Notes
The HBVcc should be handled according to the regulation of infectious agents. HBV is infectious for humans and is designated as a level 2 infectious agent. It should be handled in BSL2 facilities. All liquid and solid wastes should be disposed of after inactivation.
Acknowledgments
This protocol was adapted from our previous works: Murayama et al. (2021), Honda et al. (2021), and Washizaki et al. (2022). This work was supported by the Program on the Innovative Development and the Application of New Drugs for Hepatitis B (JP22fk0310517 and JP22fk0310503).
Competing interests
The authors declare that they have nothing to disclose.
References
- Honda, T., Yamada, N., Murayama, A., Shiina, M., Aly, H. H., Kato, A., Ito, T., Ishizu, Y., Kuzuya, T., Ishigami, M., et al. (2021). Amino acid polymorphism in hepatitis B virus associated with functional cure. Cell. Mol. Gastroenterol. Hepatol. 12(5): 1583–1598.
- König, A., Yang, J., Jo, E., Park, K. H. P., Kim, H., Than, T. T., Song, X., Qi, X., Dai, X., Park, S., et al. (2019). Efficient long-term amplification of hepatitis B virus isolates after infection of slow proliferating HepG2-NTCP cells. J. Hepatol. 71(2): 289–300.
- Murayama, A., Momose, H., Yamada, N., Hoshi, Y., Muramatsu, M., Wakita, T., Ishimaru, K., Hamaguchi, I. and Kato, T. (2019). Evaluation of in vitro screening and diagnostic kits for hepatitis B virus infection. J. Clin. Virol. 117: 37–42.
- Murayama, A., Yamada, N., Osaki, Y., Shiina, M., Aly, H. H., Iwamoto, M., Tsukuda, S., Watashi, K., Matsuda, M., Suzuki, R., et al. (2021). N‐Terminal preS1 sequence regulates efficient infection of cell‐culture–generated Hepatitis B Virus. Hepatology 73(2): 520–532.
- Otoguro, T., Tanaka, T., Kasai, H., Kobayashi, N., Yamashita, A., Fukuhara, T., Ryo, A., Fukai, M., Taketomi, A., Matsuura, Y., et al. (2020). Establishment of a cell culture model permissive for infection by hepatitis B and C viruses. Hepatology Commun. 5(4): 634–649.
- Washizaki, A., Murayama, A., Murata, M., Kiyohara, T., Yato, K., Yamada, N., Aly, H. H., Tanaka, T., Moriishi, K., Nishitsuji, H., et al. (2022). Neutralization of hepatitis B virus with vaccine-escape mutations by hepatitis B vaccine with large-HBs antigen. Nat. Commun. 13(1): e1038/s41467-022-32910-z.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Murayama, A., Akari, H. and Kato, T. (2023). Production and Purification of Cell Culture–generated Hepatitis B Virus by Transient Transfection and Density Gradient. Bio-protocol 13(14): e4779. DOI: 10.21769/BioProtoc.4779.
Category
Microbiology > Microbial biochemistry > Protein
Cell Biology > Cell-based analysis > Viral infection
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








