- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Caste Transition and Reversion in Harpegnathos saltator Ant Colonies
(*contributed equally to this work) Published: Vol 13, Iss 16, Aug 20, 2023 DOI: 10.21769/BioProtoc.4770 Views: 1450
Reviewed by: Khyati Hitesh ShahKevin Haight

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Assessing Experience-dependent Tuning of Song Preference in Fruit Flies (Drosophila melanogaster)
Xiaodong Li [...] Azusa Kamikouchi
Jul 20, 2018 7422 Views

A Method for Studying Social Signal Learning of the Waggle Dance in Honey Bees
Shihao Dong [...] Ken Tan
Aug 20, 2023 1307 Views
Habituation of Sugar-Induced Proboscis Extension Reflex and Yeast-Induced Habituation Override in Drosophila melanogaster
Swati Trisal [...] Mani Ramaswami
Dec 5, 2023 1386 Views
Abstract
Living organisms possess the ability to respond to environmental cues and adapt their behaviors and physiologies for survival. Eusocial insects, such as ants, bees, wasps, and termites, have evolved advanced sociality: living together in colonies where individuals innately develop into reproductive and non-reproductive castes. These castes exhibit remarkably distinct behaviors and physiologies that support their specialized roles in the colony. Among ant species, Harpegnathos saltator females stand out with their highly plastic caste phenotypes that can be easily manipulated in a laboratory environment. In this protocol, we provide detailed instructions on how to generate H. saltator ant colonies, define castes based on behavioral and physiological phenotypes, and experimentally induce caste switches, including the transition from a non-reproductive worker to a reproductive gamergate and vice versa (known as reversion). The unusual features of H. saltator make it a valuable tool to investigate cellular and molecular mechanisms underlying phenotypic plasticity in eusocial organisms.
Key features
• H. saltator is one of few ant species showing remarkable caste plasticity with striking phenotypic changes, being a useful subject for studying behavioral plasticity.
• Caste switches in H. saltator can be easily manipulated in a controlled laboratory environment by controlling the presence of reproductive females in a colony.
• The relatively large size of H. saltator females allows researchers to dissect various tissues of interest and conduct detailed phenotypic analyses.
Keywords: AntBackground
Eusocial insects (ants, bees, wasps, and termites) live in colonies in which all individuals have a high genetic relatedness, while only one or few individuals reproduce (Chapman et al., 2000; Trontti et al., 2005; Loope, 2015). The reproductive (queens and also kings in termites) and non-reproductive (workers) castes in a colony display different phenotypic traits including differential behavior, reproduction, metabolism, and lifespan, a phenomenon known as polyphenism (Simpson et al., 2011; Kapheim et al., 2012). Eusocial insects offer a valuable experimental model to compare gene expression between castes and to identify the key genes as causal factors determining differential phenotypes at the organismal level (Colgan et al., 2011; Bonasio et al., 2012).
Harpegnathos saltator (commonly known as the Indian jumping ant) is an ant species originating from India. These ants display an unusual feature: individuals can transition from non-reproductive workers to reproductive pseudo-queens (also called gamergates) (Liebig et al., 2000; Monnin and Peeters, 2008; Sieber et al., 2021a). When the queen is absent from a colony, workers can switch their caste and show queen-like traits: minimal movement, staying inside the nest, active ovaries, and production of eggs. In contrast, some workers show active movement in the nest to take care of the progeny, while others hunt and forage. Furthermore, workers have small ovaries and rarely produce eggs.
A colony with gamergates is as stable as a queen colony (Opachaloemphan et al., 2021; Penick et al., 2021). Additionally, gamergates can be experimentally converted back to workers (to distinguish, they will be referred to as revertants) (Penick et al., 2021). This process is associated with the reversion of phenotypic traits: revertants display worker-like physiology, behavior, and aging (Penick et al., 2021; Yan et al., 2022). With a high-quality genome (Bonasio et al., 2010; Shields et al., 2018), multiple studies have been performed in H. saltator to analyze caste-specific transcriptomes and methylomes at tissue and/or single-cell levels (Bonasio et al., 2012; Sheng et al., 2020; Sieriebriennikov et al., 2021). H. saltator is one of the three species (along with Oocerea biroi and Solenopsis invicta) in which reverse genetics was applied in ants using the CRISPR-Cas9 system (Trible et al., 2017; Yan et al., 2017; Chiu et al., 2020). This experimental paradigm has helped to identify cellular and molecular processes required for the establishment of caste identity (Gospocic et al., 2021; Opachaloemphan et al., 2021) and determine the role of neuropeptides, hormones, and transcription factors in regulating caste-specific behaviors, and the role of insulin signaling in reproduction and longevity (Penick et al., 2014; Gospocic et al., 2017 and 2021; Yan et al., 2022). Here, we provide a detailed protocol explaining the experimental framework we have used on H. saltator to induce caste transition and reversion, the identifiable and quantifiable parameters to define each caste, and the techniques for tissue dissection and staining.
Materials and reagents
Oil-based paint markers (Uni-Paint, catalog number: 63721); store at room temperature
Entomology featherweight forceps (BioQuip, Featherweight Forceps, catalog number: 4748)
Dissection forceps (Dumont #55, catalog number: 11295-51)
Wood flour (System Three Resins, System Three); store at room temperature and dry
Dental plaster (Kulzer, Modern Materials Lab Stone Blue Type III); store at room temperature
Small plastic container (Pioneer Plastics, catalog number: 028C, inner dimensions: 9.52 cm × 9.52 cm × 7.77 cm)
Medium plastic container (Pioneer Plastics, catalog number: 079C, inner dimensions: 18.89 cm × 13.49 cm × 9.53 cm)
Crickets (Ghann’s Crickets, Live Crickets " and ¼", catalog number: C14 and C38, respectively)
Cricket chow (Ghann’s Crickets, Ghann’s Cricket chow, catalog number: FD-GCC); store at room temperature. Mix with apples and potatoes for feeding crickets
Cover glass for the chamber (dimensions: 10.0 cm × 9.0 cm × 0.5 cm)
Fluon (Fluorogistx, TeflonTM PTFE DISP 30 Fluoropolymer Dispersion, catalog number: D14783090); store at room temperature
2" Poly foam brush (Jen Manufacturing, catalog number: JNSN846)
Multipurpose foam mats [American Floor Mats, Soft Floors Interlocking Tiles, dimensions: 9.0 cm × 7.5 cm × 1.0 cm with an additional area (bulge) of 2.3 cm × 1.5 cm × 1.0 cm for the nest entrance]
16% paraformaldehyde (PFA) (Electron Microscopy Sciences, catalog number: 15710); store at room temperature
Triton X-100 (Sigma, catalog number: X100); store at room temperature
Alexa FluorTM 488 Phalloidin (Thermo Fisher, Alexa FluorTM, catalog number: A12379); store at 4 °C and protected from light
DAPI dye (Tocris, catalog number: 5748); store at -20 °C
Aluminum foil (Reynolds Wrap)
Single sided iSpacer 0.5 mm deep (SubJin Lab Optical Clearing Innovation, iSpacer, catalog number: IS008)
Microscope slides (Fisher Scientific, catalog number: 12-550-143)
Microscope cover glass (Fisher Scientific, catalog number: 12-542A)
Mounting solution (Thermo Fisher, Slow-FadeTM Gold Antifade mountant, catalog number: S36936); store at room temperature and protected from light
Black dissection Petri dish (Pyrex, catalog number: DD-90-S-BLK)
Sodium chloride (NaCl) (Fisher Scientific, catalog number: S271); store at room temperature
Potassium chloride (KCl) (Fisher Scientific, catalog number: 217); store at room temperature
Sodium phosphate dibasic (Na2HPO4) (Fisher Scientific, catalog number: S374); store at room temperature
Potassium phosphate monobasic (KH2PO4) (Fisher Scientific, catalog number: P285); store at room temperature
Hydrochloric acid (HCl) (Fisher Scientific, catalog number: A144S); store at room temperature
1× PBS pH 7.4 (store at room temperature, see Recipes)
Equipment
Temperature-controlled incubator (Percival Scientific, catalog number: I-36VL)
Tube rotator (Thermo Scientific, Tube Revolver, catalog number: 11-676-341)
Software
Fiji (ImageJ, https://imagej.net/software/fiji/) (Schindelin et al., 2012)
Procedure
Nest preparation
Prepare a mix of 1,200 mL of deionized H2O and 1,900 g of dental plaster powder. Add the powder gradually while stirring. Dissolve any clumps and avoid forming bubbles. Add a small quantity of plaster powder or water to achieve the best consistency. This quantity is enough to prepare four medium-sized (Figure 1A) or eight small containers.
Pour the mix into the containers, forming a layer 2.5 cm high for medium containers (Figure 1B) or 1.5 cm for small containers. Remove any air bubbles after pouring by tapping the bottoms of the containers against a solid surface (Sieber et al., 2021b). This needs to be done right after pouring the mix, as it does not take long to solidify.
While the wet plaster is drying, place a cover glass on top of a foam mat and place them near the rear center of the nest (Figure 1C). Push down the mat a few centimeters deep into the plaster to form the chamber (only for medium containers, Figure 1D) (Sieber et al., 2021b). The cover glass should not be submerged into the plaster, and the mat should not reach the bottom of the plastic container.
On the next day, remove the cover glass and the mat using a spatula. Use the spatula to remove extra plaster and make uniform borders around the chamber. Leave the nest to dry for one week.
Once the nest is dry, prepare a solution with equal parts Fluon and H2O together. Mix by gently swirling to avoid bubbles. Hold the nest upside down and use a foam brush to apply a continuous layer throughout all the walls of the container, starting 1 inch above the plaster surface (Figure 1D). Avoid bubbles and contact between Fluon and the plaster. Leave the nest to dry by placing it upside down.
After Fluon dries, place the cover glass over the chamber (Figure 1B and 1D). Place the lid on the container. The nest is now ready to use. Alternatively, store the nest at room temperature.
Note: Use the same procedure for small containers. Skip step A3 and A4.
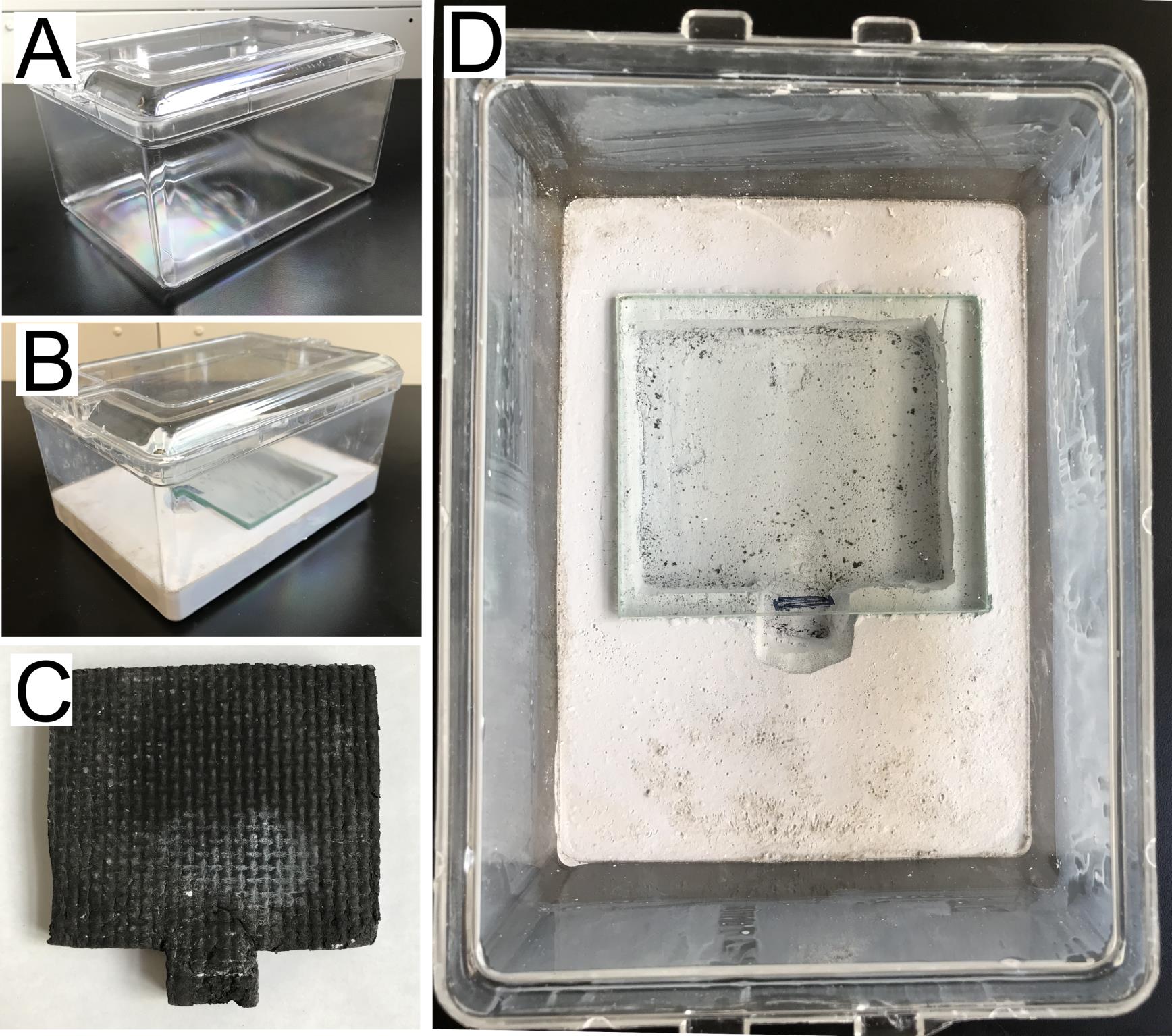
Figure 1. Nest preparation. (A) Plastic containers are used to make ant nests. (B) Dental plaster is used to make the nest in the medium plastic container forming a 2.5 cm thick layer with a deep area for the chamber in the center. (C) A foam mat (see Materials and Reagents) is used during nest preparation to cast the chamber in the plaster. (D) A cover glass is used to cover the chamber. A layer of Fluon is applied to the walls of the container, which prevents the ants from crawling out.
Caste transition (from workers to gamergates) and caste reversion (from gamergates to revertants)
Generate gamergates: set up a transition colony containing 30 age-matched workers (approximately 2–4 weeks post eclosion) reared in a temperature-controlled incubator (approximately 25 °C).
Collect 30 young workers (a.k.a. callows) that can be distinguished by their yellow cuticle in contrast to the brown cuticle of older workers (Figure 2A). They should be collected from a healthy and mature colony, which contains eggs, larvae, pupae, at least 100 adults, and, importantly, contains no dueling or policing events.
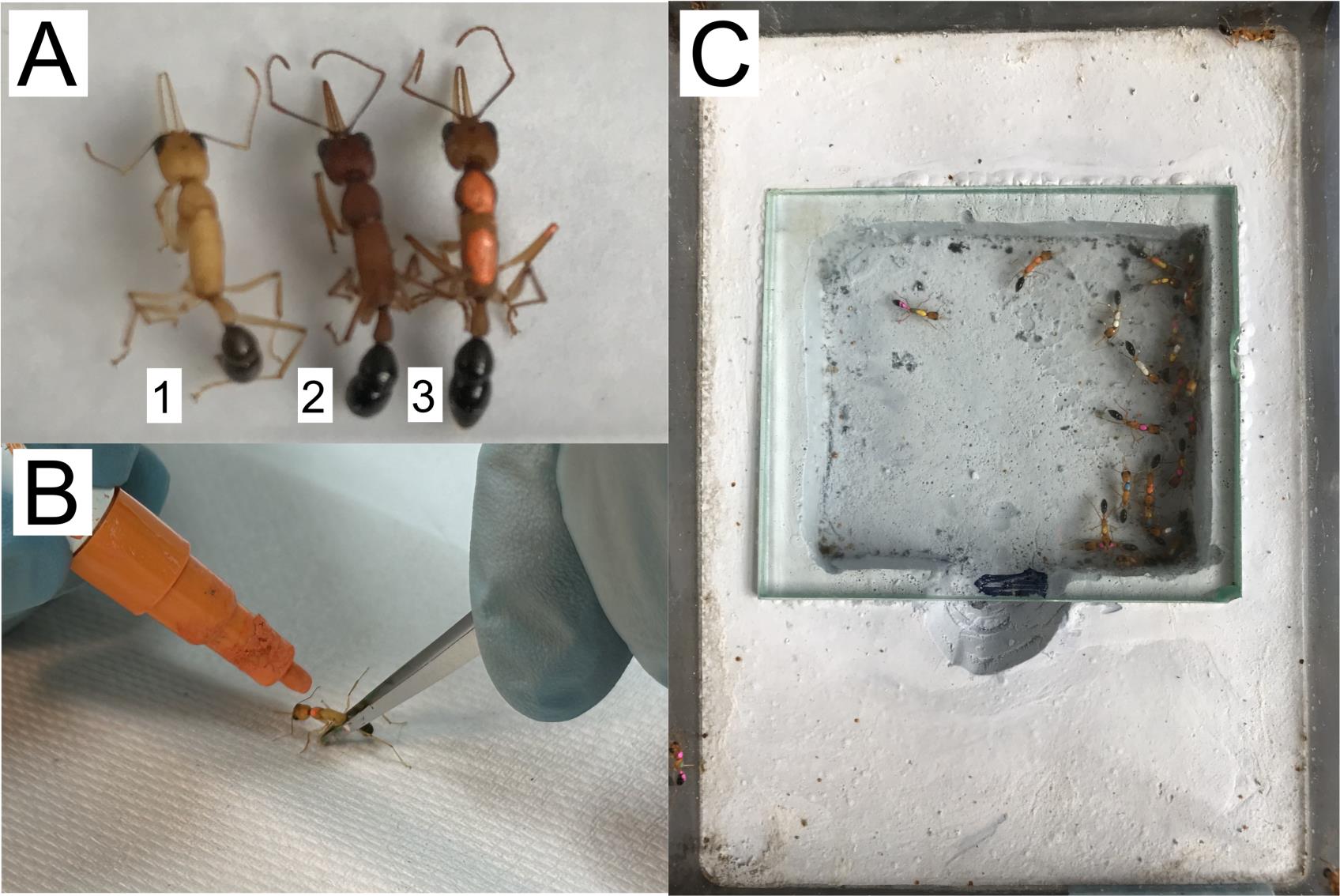
Figure 2. Paint marks to identify ants. (A) Recently eclosed ants, also called callows (1), are identified by their yellow cuticle, as opposed to mature ants with dark brown cuticle (2). Oil-based paint markers are used to label each ant with a unique combination of colors (3). (B) Marks are painted while holding the ants with featherweight forceps. (C) The transition colony is set with 30 workers, each marked with a unique combination of colors.Label each callow with oil-based paint markers. Gently hold the ant by the thorax using featherweight forceps and paint each ant with a dot of different color on its thorax. Some ants can be painted with two or three dots, so the combination of colors can be used to distinguish individuals (Figure 2B).
Note: Handle callows gently, as they have a very soft cuticle that can be easily damaged.
Keep the labeled callows in an empty box for at least 5 min for the paint to dry before returning them back to their original colony. Keep them in that colony for one week.
Prepare a new medium-sized nest box by adding deionized water to wet the plaster, as mineral ions in water can affect the plaster’s absorption ability. Watering the plaster is necessary to maintain humidity in the colony. Wait for water to be absorbed. Transfer the labeled callows to the new nest (Figure 2C).
Behavioral observations during the transition from worker to gamergate: to distinguish the prospective gamergates from workers, we monitor for gamergate-specific behaviors, such as antennal dueling, egg-laying, and dominance (see below). On the other hand, workers perform foraging, cleaning, and colony defense.
Feed with five pre-stung crickets (previously stung crickets by workers of another mature colony) per transition colony every two days or three times per week.
While feeding, remove rotten crickets and any colony waste.
Observe the frequency of antennal dueling for at least 15 min each time, three times per week (Figure 3A and Video 1). The first observation of dueling typically occurs within the first 3–7 days after the colony is set up. This is counted as day 1 of the worker-to-gamergate transition.
Note: Intensive and consistent dueling behavior for at least one week is an early indication of the prospective gamergate. Use their painted color code to track gamergate- and worker-specific behaviors of all individuals in the colony. Individuals that show foraging behavior (stinging crickets and moving them into the chamber) and no dueling are workers.
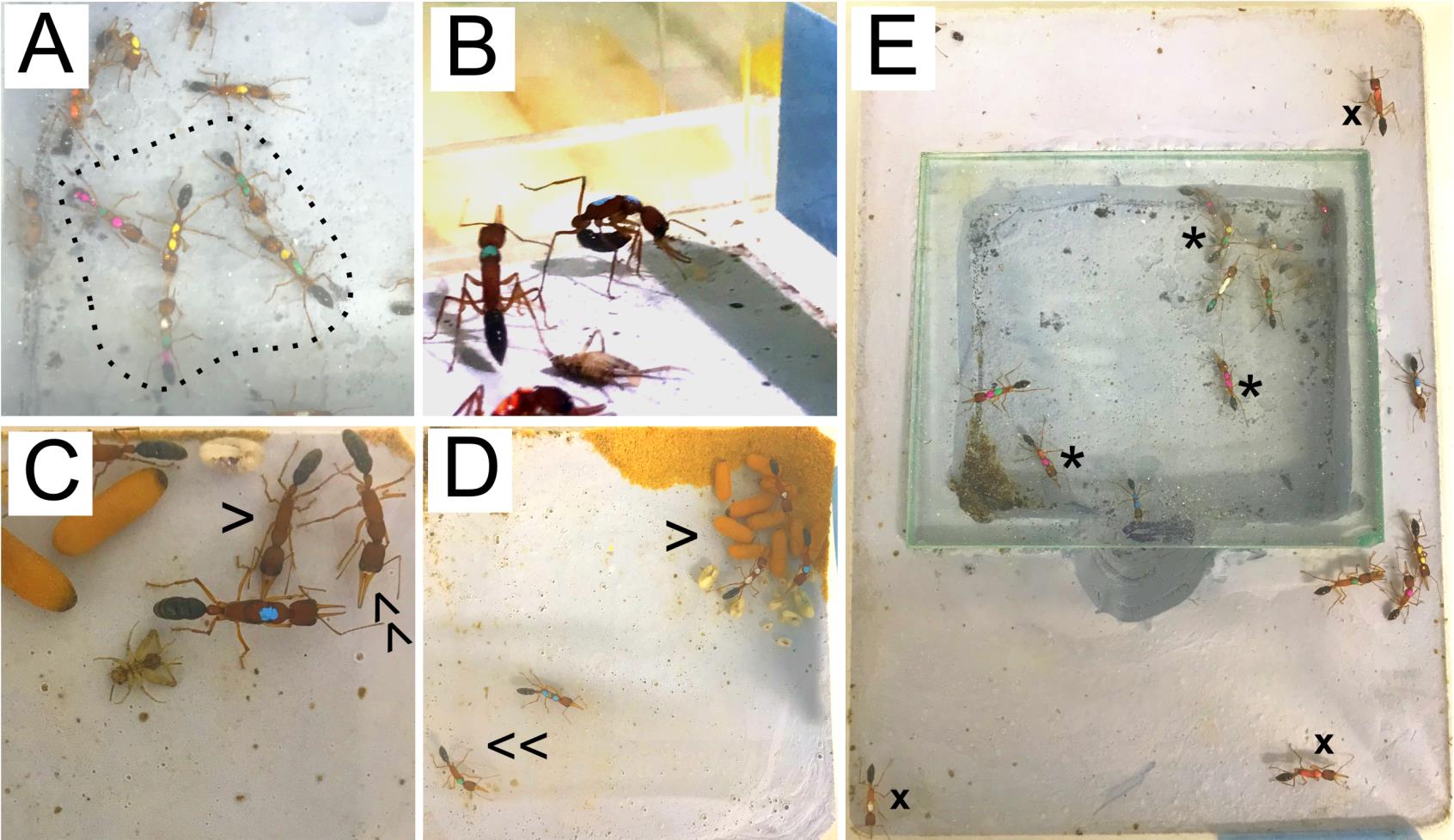
Figure 3. Differential phenotypes of prospective gamergates and workers during transition. (A) Constant duelers actively interact between themselves, forming a gamergate circle (dashed line). (B) Egg laying can be identified by ants bending their gaster towards their head, staying in this position for several minutes. (C) A worker approaches a gamergate (blue mark) with its body laid down close to the ground in a submissive position (single arrowhead). Compare this with the stance of another worker (double arrowhead) that is not showing a submissive position. (D) Gamergates are usually found on top of the pupae (single arrowhead) and are less active than workers (double arrowhead). (E) Workers can be found either inside (*) or outside (x) the chamber, while gamergates are exclusively found inside the chamber.Video 1. Antennal dueling during the caste transition from workers (W) to gamergates (G). Note that the dueling occurred between five ants near the center of the chamber, marked with yellow-green, green-green, yellow-yellow, white-green-pink, and pink-green-pink.Observe egg-laying events in the dueling ants: dueling ants begin laying eggs approximately one week after initiating the transition (Figure 3B). Constant egg-laying during the first three months of caste transition is another indication of mature gamergates. The prospective gamergate also displays dominant behavior: standing tall when confronting a non-reproductive worker, while the submissive worker keeps its head low (Figure 3C). In addition, gamergates are frequently found on the top of the brood pile and located inside the nest (Figure 3D and 3E). In a colony of 30 ants, 3–6 gamergates are expected to be identified.
The worker-to-gamergate transition normally takes three months.
Caste reversion (from gamergates to revertants): this step is to revert mature gamergates obtained from the previous caste transition back to a non-reproductive status (Figures 4 and 5).
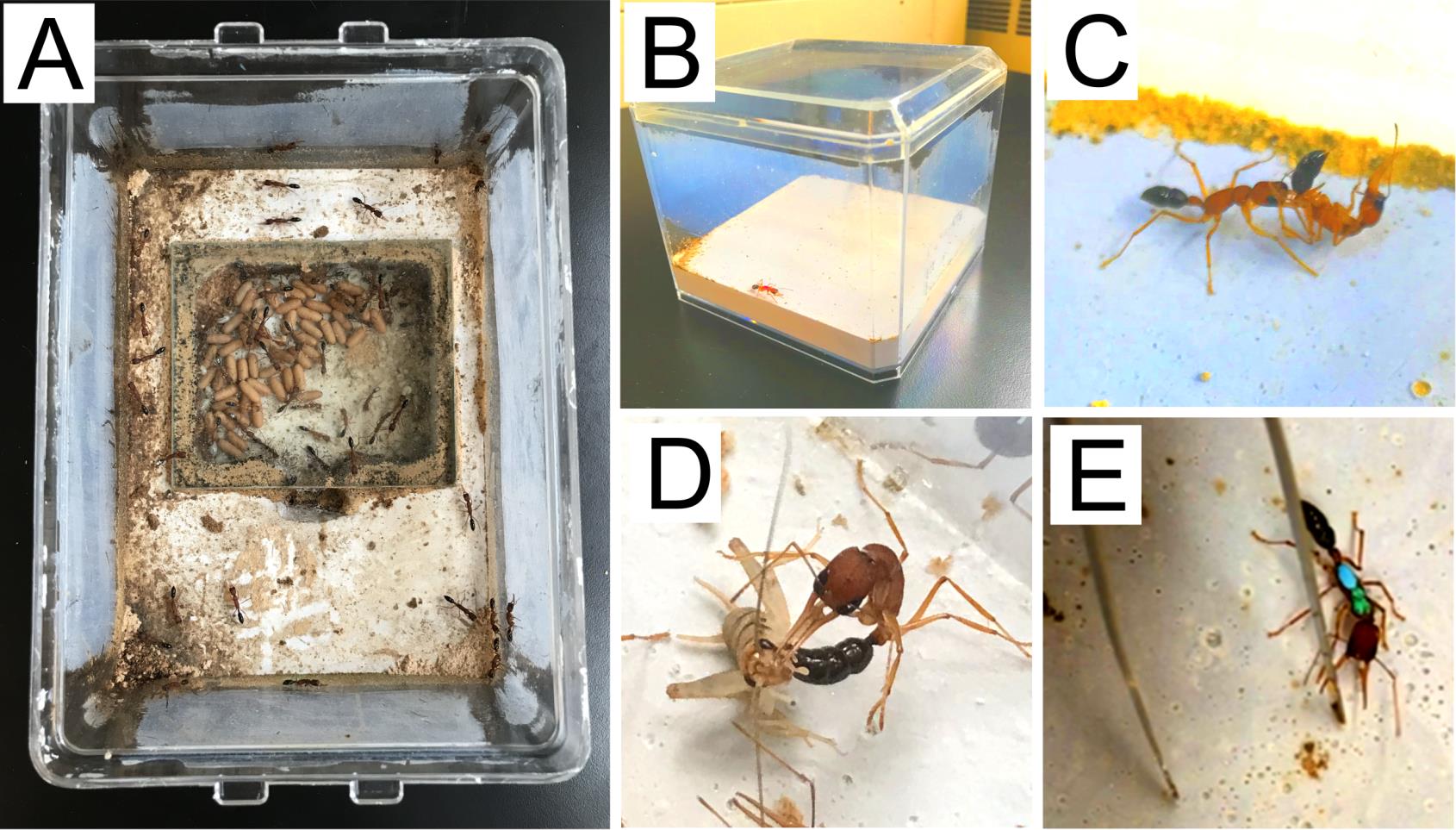
Figure 4. Caste reversion. (A) A mature and stable colony is used as a host colony (see diagram in Figure 5). (B) Gamergates are isolated individually in a small nest box prior to policing. (C) Policing is usually observed against isolated gamergates when they are transferred into the host colony. (D) Revertants show worker phenotypes as hunting and stinging crickets. (E) Revertants display an aggressive response when being provoked by featherweight forceps.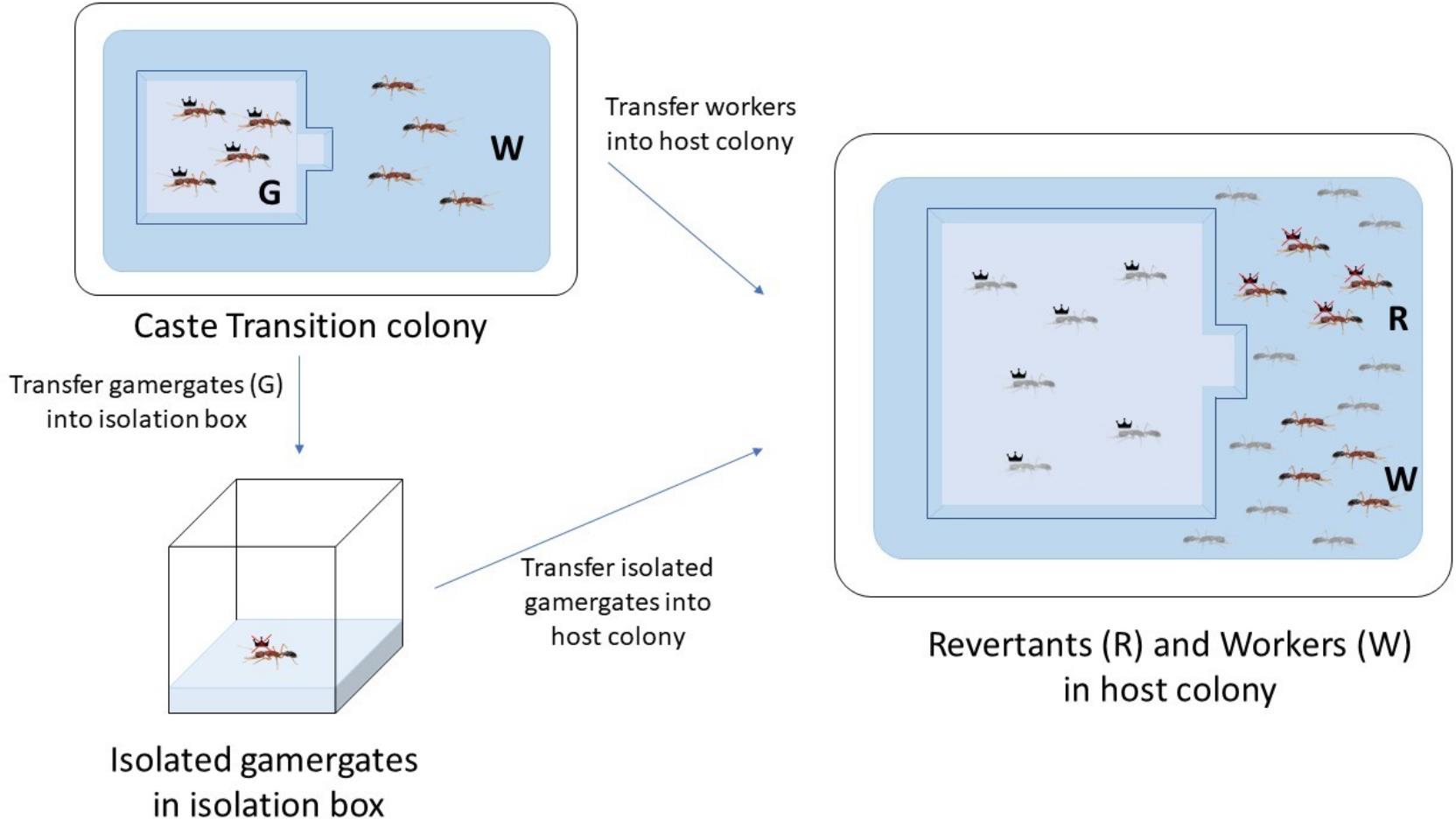
Figure 5. Diagram of caste reversion from gamergates (G) to revertants (R). Four gamergates and four workers (W) are collected from the caste transition colony. Four workers are transferred to the host colony. The gamergates are first individually isolated in a small nest box for four weeks, after which they are transferred to the host colony. Light-gray colored ants indicate local gamergates and workers from the host colony, while dark-colored ants represent gamergates and workers transferred for reversion.From the transition colony, choose four mature gamergates with the most frequent dueling and egg laying events. These gamergates are subject to individual isolation followed by their reversion in another healthy and stable colony (referred now as a host colony in Figure 4A). Choose four workers with the least dueling and no egg-laying events and directly transfer them into the host colony (Figure 5).
Behavioral observations:
Prepare small nest boxes similar to the method of making medium-sized nests, but do not cast a chamber. Add some deionized water to provide humidity to the box. Transfer the gamergates selected in step B3a out of the transition colony and isolate each gamergate in a small nest box (Figure 4B). Add 2–3 small pre-stung crickets and a pinch of wood flour (sawdust), which is used by workers for cleaning and brood rearing tasks. This individual isolation reduces the fertility status due to the lack of social interaction (Penick et al., 2021). During the first week of isolation, gamergates still lay eggs, which helps to confirm their gamergate status. Keep the gamergates isolated for four weeks. The egg-laying events will slowly fade out. Feed them by adding new crickets three times per week.
While step B3b starts, transfer four workers selected in step B3a to the host colony, where these four workers will maintain their worker status.
After four weeks of isolation, transfer each gamergate to the host colony (the same host colony that is used in step B3c). Policing is exerted by workers from the host colony towards the recently transferred gamergates (Liebig et al., 1999; Penick et al., 2021) (Figure 4C). On the contrary, policing events rarely occur towards the workers transferred in step B3c.
After two months, test the behavior of transferred gamergates (now revertants, see below). The revertants stop laying eggs, show foraging behavior (Figure 4D), and spend more time outside the chamber as workers (Figure 3E).
Testing revertants for worker-like behaviors:
Cricket stinging test: place one revertant with one live cricket in an empty nest and test for hunting behavior. When the revertant hunts, it grabs the cricket with its mandibles and stings the cricket with its stinger (Figure 4D). After being stung, the cricket gets paralyzed.
Forceps assay: place a revertant in an empty nest and leave it there for 10 min to acclimate. Then, use featherweight forceps to provoke the revertant by rapidly snapping the forceps in front of their antennae. The revertant aggressively bites or grabs the forceps with its mandible (Figure 4E).
Dissection and staining
Ovary tissue dissection:
Cool 1× PBS by placing it on ice. Place the ant to be dissected in a 1.5 mL tube on ice for a few minutes to let it fall asleep.
Make a cut with scissors in the narrow region (petiole) that connects the gaster with the thorax.
Take the gaster to the black dissection Petri dish. Place it in a drop of ice-cold 1× PBS. For workers, use dissection forceps to pull the stinger out with a single, quick movement. This will take out the gut and other attached tissues, ovaries included. Avoid using this technique for gamergates, as it causes the oocytes to burst. Instead, open the gaster with dissection forceps by pulling apart between the second and third abdominal segments (tergites). Do not insert the tip of the forceps deep into the gaster to avoid damaging the ovaries.
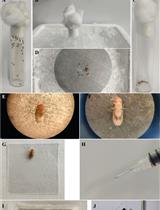
Find the ovaries close to the stinger, ventral to the gut. They look like two structures bound together at the base (Figure 6A and 6B). If it is a gamergate, oocytes are evident. Be careful in this case, as large oocytes can be easily burst (Figure 6B).
Use the dissection forceps to separate the ovaries from the rest of the tissues. Do not break the gut, as the midgut and the rectum will release their contents if burst and will damage surrounding tissues. Fat body cells are usually found attached to the ovaries and should be removed.
Move the isolated ovaries to a separate drop of 1× PBS. The worker ovaries are formed by two groups of four ovarioles and look transparent (Figure 6A). The gamergate ovaries contain egg chambers in different stages of development and can be distinguished by their white yolk and oval shape (Figure 6B).
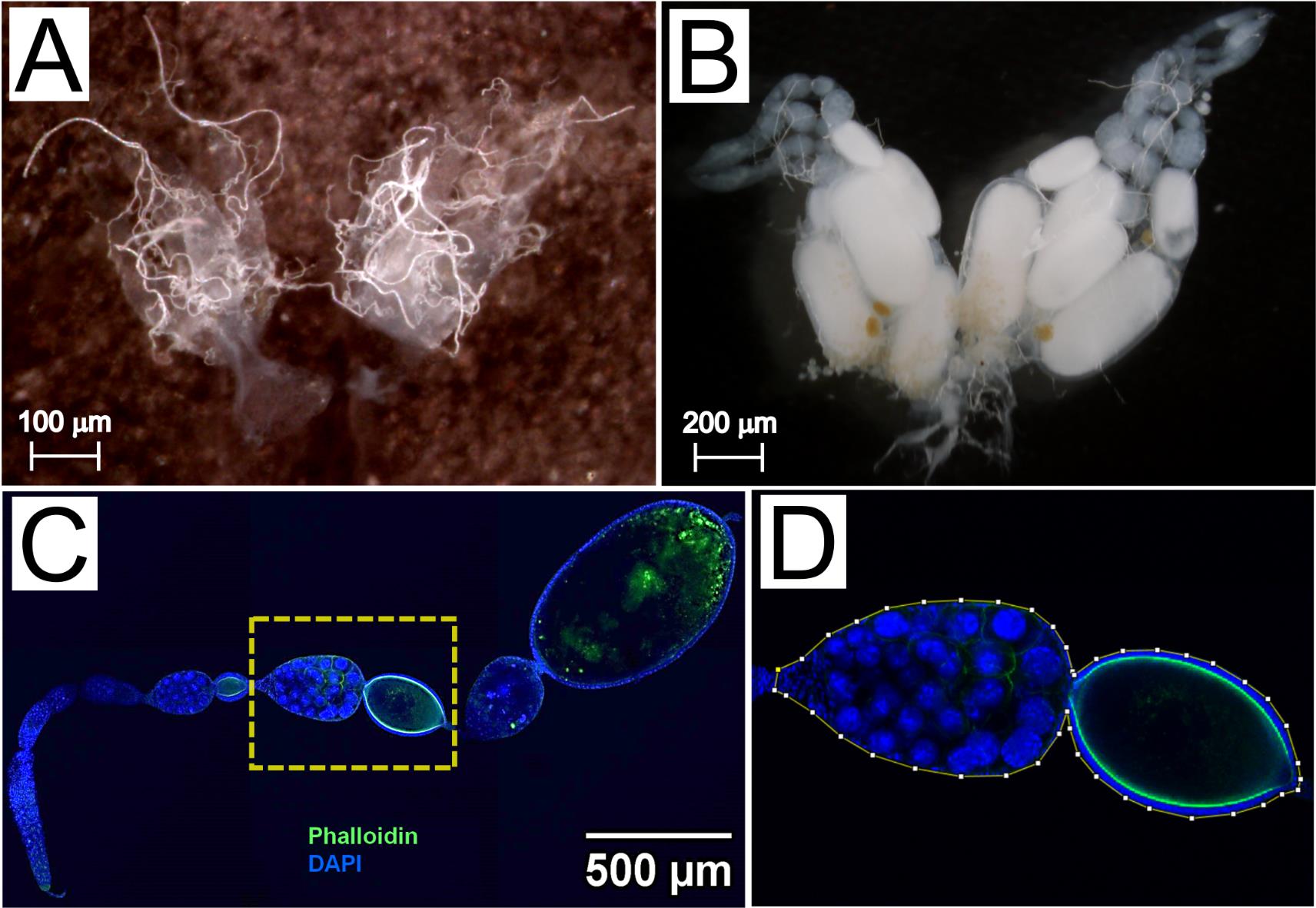
Figure 6. Ovary dissection and immunofluorescence staining. (A) Brightfield image of worker ovaries, with four transparent ovarioles on each side. Scale bar, 100 μm. (B) Brightfield image of gamergate ovaries showing egg chambers in different developmental stages. The most mature oocyte has an oval shape and contains a dense white yolk. Scale bar, 200 μm. (C) Confocal image of a gamergate ovariole stained with Phalloidin (green) and DAPI (blue). Scale bar, 500 μm. (D) A yellow line is drawn in Fiji (see Data Analysis) around the borders of an egg chamber to measure its area (magnified from C).Immunofluorescent staining:
Fix the dissected ovaries by adding 1 mL of freshly prepared 4% PFA in 1× PBS in a 1.5 mL tube.
Rotate the fixing tissues for 30 min at room temperature or overnight at 4 °C on a tube rotator.
Remove the fixation solution and then wash the tissue with 1 mL of 0.1% Triton X-100 in 1× PBS (PBSTx). Incubate on a rotator for 15 min.
Repeat step C2c three times.
Remove PBSTx and add 1 mL of PBSTx containing 2 μg/mL DAPI and 1:100 Alexa FluorTM 488 Phalloidin. DAPI and Phalloidin staining detect the nuclei and cell shape, respectively.
Protect the tube from light by covering it with aluminum foil.
Rotate the tube overnight at 4 °C on a rotator.
Remove the solution and replace it with 1 mL of PBSTx.
Rotate the tube for 5 min.
Repeat step C2h and C2i five times.
Cut a pipette tip with scissors to leave a wide-opening tip.
Prepare a glass slide by attaching a 0.5 mm iSpacer on the slide. This prevents the tissue from being smashed after placing the glass cover.
Transfer the tissue to the iSpacer slide from the previous step. Use the wide-opening pipette tip prepared in step C2k to minimize any damage to the tissue.
Remove any excess solution from the iSpacer using a pipette.
Add 20 μL of the Slow-FadeTM mounting solution.
Place the glass cover and seal the edges with clear nail polish.
Air-dry the nail polish for approximately 5 min. Protect it from light.
Keep the slide at 4 °C in the slide tray. Take images with a confocal microscope.
Data analysis
Image analysis
To measure the area of the mature egg chambers, use the Fiji software (Schindelin et al., 2012).
The ovary is a relatively thick tissue. Z-stack confocal images are normally taken to inspect the whole tissue.
Choose the section through the Z-stack that contains the largest surface area of the egg chamber to be measured.
Select Analyze from the menu bar and then Set Scale. A window will pop up, displaying the Scale. Adjust accordingly.
Use the Polygon tool under the menu bar to draw a line that surrounds the egg chamber to be measured as shown in Figure 6C and 6D. An egg chamber is formed by the nurse cells and the oocyte.
Note: For a more accurate drawing, use the Magnifying glass tool under the menu bar to zoom in on the image.
Select Analyze/Measure from the menu bar. The measured surface area appears in a pop-up window.
Recipes
1× PBS pH 7.4
Reagent Final concentration NaCl 137 mM KCl 2.7 mM Na2HPO4 4.3 mM KH2PO4 1.4 mM Adjust the pH to 7.4 with HCl H2O add up to 1,000 mL
Acknowledgments
This protocol was derived from the research published in Yan et al. (2022). This work was supported by HHMI Collaborative Innovation Award (CIA) #2009005 and HCIA #2009005, NIH grants R21GM114457, R01EY13010, R01AG058762, R01DC020203, NIH Ruth L. Kirschstein NRSA postdoctoral fellowship F32AG044971 and NSF I/UCRC CAMTech grant IIP1821914. We thank Jakub Mlejnek for technical support, and Kayli Sieber for help with manuscript preparation.
Competing interests
The authors have no competing interests to declare.
References
- Bonasio, R., Li, Q., Lian, J., Mutti, N. S., Jin, L., Zhao, H., Zhang, P., Wen, P., Xiang, H., Ding, Y., et al. (2012). Genome-wide and Caste-Specific DNA Methylomes of the Ants Camponotus floridanus and Harpegnathos saltator. Curr. Biol. 22(19): 1755–1764.
- Bonasio, R., Zhang, G., Ye, C., Mutti, N. S., Fang, X., Qin, N., Donahue, G., Yang, P., Li, Q., Li, C., et al. (2010). Genomic Comparison of the Ants Camponotus floridanus and Harpegnathos saltator. Science 329(5995): 1068–1071.
- Chapman, T. W., Crespi, B. J., Kranz, B. D. and Schwarz, M. P. (2000). High relatedness and inbreeding at the origin of eusociality in gall-inducing thrips. Proc. Natl. Acad. Sci. U.S.A. 97(4): 1648–1650.
- Chiu, Y. K., Hsu, J. C., Chang, T., Huang, Y. C. and Wang, J. (2020). Mutagenesis mediated by CRISPR/Cas9 in the red imported fire ant, Solenopsis invicta. Insectes Soc. 67(2): 317–326.
- Colgan, T. J., Carolan, J. C., Bridgett, S. J., Sumner, S., Blaxter, M. L. and Brown, M. J. (2011). Polyphenism in social insects: insights from a transcriptome-wide analysis of gene expression in the life stages of the key pollinator, Bombus terrestris. BMC Genomics 12(1): e1186/1471-2164-12-623.
- Gospocic, J., Glastad, K. M., Sheng, L., Shields, E. J., Berger, S. L. and Bonasio, R. (2021). Kr-h1 maintains distinct caste-specific neurotranscriptomes in response to socially regulated hormones. Cell 184(23): 5807–5823.e14.
- Gospocic, J., Shields, E. J., Glastad, K. M., Lin, Y., Penick, C. A., Yan, H., Mikheyev, A. S., Linksvayer, T. A., Garcia, B. A., Berger, S. L., et al. (2017). The Neuropeptide Corazonin Controls Social Behavior and Caste Identity in Ants. Cell 170(4): 748–759.e12.
- Kapheim, K. M., Smith, A. R., Nonacs, P., Wcislo, W. T. and Wayne, R. K. (2012). Foundress polyphenism and the origins of eusociality in a facultatively eusocial sweat bee, Megalopta genalis (Halictidae). Behav. Ecol. Sociobiol. 67(2): 331–340.
- Liebig, J., Peeters, C. and Hölldobler, B. (1999). Worker policing limits the number of reproductives in a ponerine ant. Proc. Royal Soc. B 266(1431): 1865–1870.
- Liebig, J., Peeters, C., Oldham, N. J., Markstädter, C. and Hölldobler, B. (2000). Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator ?. Proc. Natl. Acad. Sci. U.S.A. 97(8): 4124–4131.
- Loope, K. J. (2015). Queen Killing Is Linked to High Worker-Worker Relatedness in a Social Wasp. Curr. Biol. 25(22): 2976–2979.
- Monnin, T. and Peeters, C. (2008). How many gamergates is an ant queen worth?. Naturwissenschaften 95(2): 109–116.
- Opachaloemphan, C., Mancini, G., Konstantinides, N., Parikh, A., Mlejnek, J., Yan, H., Reinberg, D. and Desplan, C. (2021). Early behavioral and molecular events leading to caste switching in the ant Harpegnathos. Genes Dev. 35: 410–424.
- Penick, C. A., Brent, C. S., Dolezal, K. and Liebig, J. (2014). Neurohormonal changes associated with ritualized combat and the formation of a reproductive hierarchy in the ant Harpegnathos saltator. J. Exp. Biol. 217(9): 1496–1503.
- Penick, C. A., Ghaninia, M., Haight, K. L., Opachaloemphan, C., Yan, H., Reinberg, D. and Liebig, J. (2021). Reversible plasticity in brain size, behaviour and physiology characterizes caste transitions in a socially flexible ant (Harpegnathos saltator). Proc. Royal Soc. B 288(1948): e0141.
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9(7): 676–682.
- Sheng, L., Shields, E. J., Gospocic, J., Glastad, K. M., Ratchasanmuang, P., Berger, S. L., Raj, A., Little, S. and Bonasio, R. (2020). Social reprogramming in ants induces longevity-associated glia remodeling. Sci. Adv. 6(34): eaba9869.
- Shields, E. J., Sheng, L., Weiner, A. K., Garcia, B. A. and Bonasio, R. (2018). High-Quality Genome Assemblies Reveal Long Non-coding RNAs Expressed in Ant Brains. Cell Rep. 23(10): 3078–3090.
- Sieber, K. R., Dorman, T., Newell, N. and Yan, H. (2021a). (Epi)Genetic Mechanisms Underlying the Evolutionary Success of Eusocial Insects. Insects 12(6): 498.
- Sieber, K., Saar, M., Opachaloemphan, C., Gallitto, M., Yang, H. and Yan, H. (2021b). Embryo Injections for CRISPR-Mediated Mutagenesis in the Ant Harpegnathos saltator. J. Vis. Exp. (168): e61930.
- Sieriebriennikov, B., Reinberg, D. and Desplan, C. (2021). A molecular toolkit for superorganisms. Trends Genet. 37(9): 846–859.
- Simpson, S. J., Sword, G. A. and Lo, N. (2011). Polyphenism in Insects. Curr. Biol. 21(18): R738-749.
- Trible, W., Olivos-Cisneros, L., McKenzie, S. K., Saragosti, J., Chang, N. C., Matthews, B. J., Oxley, P. R. and Kronauer, D. J. (2017). orco Mutagenesis Causes Loss of Antennal Lobe Glomeruli and Impaired Social Behavior in Ants. Cell 170(4): 727–735.e10.
- Trontti, K., Aron, S. and Sundstrom, L. (2005). Inbreeding and kinship in the ant Plagiolepis pygmaea. Mol. Ecol. 14(7): 2007–2015.
- Yan, H., Opachaloemphan, C., Carmona-Aldana, F., Mancini, G., Mlejnek, J., Descostes, N., Sieriebriennikov, B., Leibholz, A., Zhou, X., Ding, L., et al. (2022). Insulin signaling in the long-lived reproductive caste of ants. Science 377(6610): 1092–1099.
- Yan, H., Opachaloemphan, C., Mancini, G., Yang, H., Gallitto, M., Mlejnek, J., Leibholz, A., Haight, K., Ghaninia, M., Huo, L., et al. (2017). An Engineered orco Mutation Produces Aberrant Social Behavior and Defective Neural Development in Ants. Cell 170(4): 736–747.e9.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Opachaloemphan, C., Carmona-Aldana, F. and Yan, H. (2023). Caste Transition and Reversion in Harpegnathos saltator Ant Colonies. Bio-protocol 13(16): e4770. DOI: 10.21769/BioProtoc.4770.
- Yan, H., Opachaloemphan, C., Carmona-Aldana, F., Mancini, G., Mlejnek, J., Descostes, N., Sieriebriennikov, B., Leibholz, A., Zhou, X., Ding, L., et al. (2022). Insulin signaling in the long-lived reproductive caste of ants. Science 377(6610): 1092–1099.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









