- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Ex vivo Culture and Contractile Force Measurements of Non-human Primate Heart Slices
Published: Vol 13, Iss 13, Jul 5, 2023 DOI: 10.21769/BioProtoc.4750 Views: 1488
Reviewed by: Ralph Thomas BoettcherDavide BottaFarah Haque

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
An in vitro DNA Sensor-based Assay to Measure Receptor-specific Adhesion Forces of Eukaryotic Cells and Pathogens
Maurizio Wack [...] E. Ada Cavalcanti-Adam
Sep 5, 2020 5652 Views
Catheterization of Pulmonary and Carotid Arteries for Concurrent Measurement of Mean Pulmonary and Systemic Arterial Pressure in Rat Models of Pulmonary Arterial Hypertension
Tanoy Sarkar [...] Fakhrul Ahsan
Aug 20, 2023 1768 Views
An Ex Vivo Lung Histoculture Model for Studying Pulmonary Infection and Immune Response With SARS-CoV-2 as an Example of RNA Virus
Elena V. Maryukhnich [...] Elena J. Vasilieva
Dec 20, 2025 737 Views
Abstract
Cardiovascular diseases are the leading cause of death and morbidity worldwide. Patient mortality has been successfully reduced by nearly half in the last four decades, mainly due to advances in minimally invasive surgery techniques and interventional cardiology methods. However, a major hurdle is still the translational gap between preclinical findings and the conversion into effective therapies, which is partly due to the use of model systems that fail to recapitulate key aspects of human physiology and disease. Large animal models such as pigs and non-human primates are highly valuable because they closely resemble humans but are costly and time intensive. Here, we provide a method for long-term ex vivo culture of non-human primate (NHP) myocardial tissue that offers a powerful alternative for a wide range of applications including electrophysiology studies, drug screening, and gene function analyses.
Graphical overview
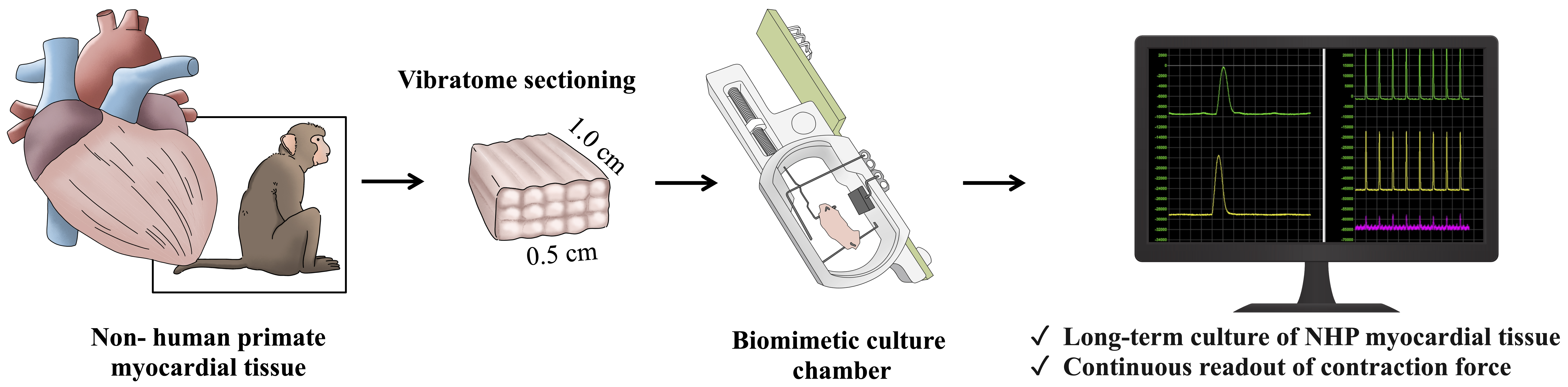
Background
Cells isolated from animal or human hearts by enzymatic dissociation have provided fundamental insights into the electrophysiology, contractile function, and transcriptional profiles of cardiomyocytes (Liu et al., 2021). However, cells cultured in 2D lack the native environment of the 3D myocardium, which includes intra- and intercellular interactions and syncytial properties that are determinant factors in studying cells, genes, and drug modulations in cardiac pathologies. It is also known that functional and structural preservation of native myocardial tissue ex vivo is dependent on the application of both mechanical preload and electrical stimulation (Brandenburger et al. 2012; Watson et al., 2017; Perbellini et al., 2018; Qiao et al., 2019). Recently, Fischer et al. (2019) established and commercialized a biomimetic ex vivo culture system taking these parameters into account to maintain intact native human myocardial slices in culture for up to four months (Fischer et al., 2019; www.invitrosys.com). This novel technology enables human disease modeling with high temporal and spatial resolution in assessing cellular behavior after pharmacologic or genetic interventions. A drawback of this method is that human tissue is not readily available, with samples generally originating from end-stage heart failure patients with a wide range of comorbidities. Moreover, the patients’ age, genetic background, and medication can be confounding factors during ex vivo analyses. To enable standardized investigational studies, we adapted this system to the culture of non-human primate (NHP) heart slices.
NHP hearts are an excellent surrogate to address basic research questions due to their genetic, metabolic, and physiologic similarities to humans and the ability to control diet, environment, and breeding (Cox et al. 2017). As described in the step-by-step protocol below, left ventricular transmural sections of native NHP hearts are cut into thin tissue slices (300 μm) that are subsequently subjected to physiological preload and electrical stimulation. Under biomimetic culture conditions, a continuous readout of contractile performance and electrophysiologic behavior such as excitability, force-frequency relationship, and effective refractory period can be reached for up to three weeks. Furthermore, as we recently reported, this system can be used as a platform to study critical steps of cardiac regeneration processes in single-cell resolution demonstrating that time- and resource-consuming in vivo studies in animals can be partially bridged (Poch et al. 2022).
Materials and reagents
35 mm Petri dish (Corning Life Sciences, catalog number: CLS430165)
50 mL capped Falcon tube (Corning Life Sciences, catalog number: 352070)
Medium 199, Earle’s salts (M199) (Gibco, catalog number: 11150059)
Insulin-transferrin-selenium (ITS) (Gibco, catalog number: 41400045)
Penicillin-streptomycin (P/S) (Gibco, catalog number: 15140122)
β-Mercaptoethanol (Bio-Rad, catalog number: 1610747)
Histoacryl tissue glue (Braun, catalog number: 04929052)
PET plastic triangles (InVitroSys, Germany)
Isopropyl alcohol (Fischar, catalog number: 08819076)
NaCl (Sigma, catalog number: S5886-1kg)
KCl (Merck, catalog number: 1.04933.0500)
MgCl2·6H2O (AppliChem, catalog number: A1036,0500)
NaH2PO4·H2O (Merck, catalog number: 1.06346.0500)
Glucose·H2O (AppliChem, catalog number: A3730,0500)
CaC12·2H2O (Merck, catalog number: 2382.1000)
BDM (2,3-Butanedione monoxime) (Sigma-Aldrich, catalog number: B0753)
HEPES (AppliChem, catalog number: A 1069,0500)
Filtration unit (Steritop Quick Release, Millipore, S2GPTOSRE)
Agarose low melt (Carl Roth, catalog number: 6351.2)
Cutting buffer (see Recipes)
4% agarose preparation (see Recipes)
Culture medium M199 (see Recipes)
Equipment
Vibratome VT1200S (Leica Biosystems, Germany)
Vibrocheck device (Leica Biosystems, Germany)
MyoDish 1 Tissue Culture System (InVitroSys, Germany)
Incubator (37 °C, 5% CO2) (Thermo Scientific, catalog number: 4110)
Water bath (37 °C)
Razor blade (Wilkinson Sword)
Scalpel (Feather, catalog number: 00636494)
Tweezer (Mmobil®, catalog numbers: ESD-15 and ESD-10)
Software
MyoDish Software (InVitroSys, www.invitrosys.com)
MyoDish Data File Converter v1.1 (inVitroSys, www.invitrosys.com)
LabChart Reader (v8.1.24, www.adinstruments.com)
Microsoft Excel 2019 (www.microsoft.com)
GraphPad Prism 9 (www.graphpad.com)
Procedure
Preparation and assembly of MyoDish culture chambers
Prepare the cutting buffer, 4% agarose, and M199 medium as listed in the Recipes section below.
Sterilize electrodes of MyoDish Culture system (autoclave or place in isopropyl alcohol).
Prepare plastic triangles by detaching them from the holding sheet. Sterilize by autoclaving or submersion in isopropyl alcohol.
Note: Triangles can be purchased from InVitroSys or manually prepared from a foil of polyethylene terephthalate (PET, 125 μm thick).
Clean chambers with isopropyl alcohol and let them dry under a laminar flow hood.
Assemble chambers and electrodes according to manufacturer’s instructions (www.invitrosys.com; Figure 1).
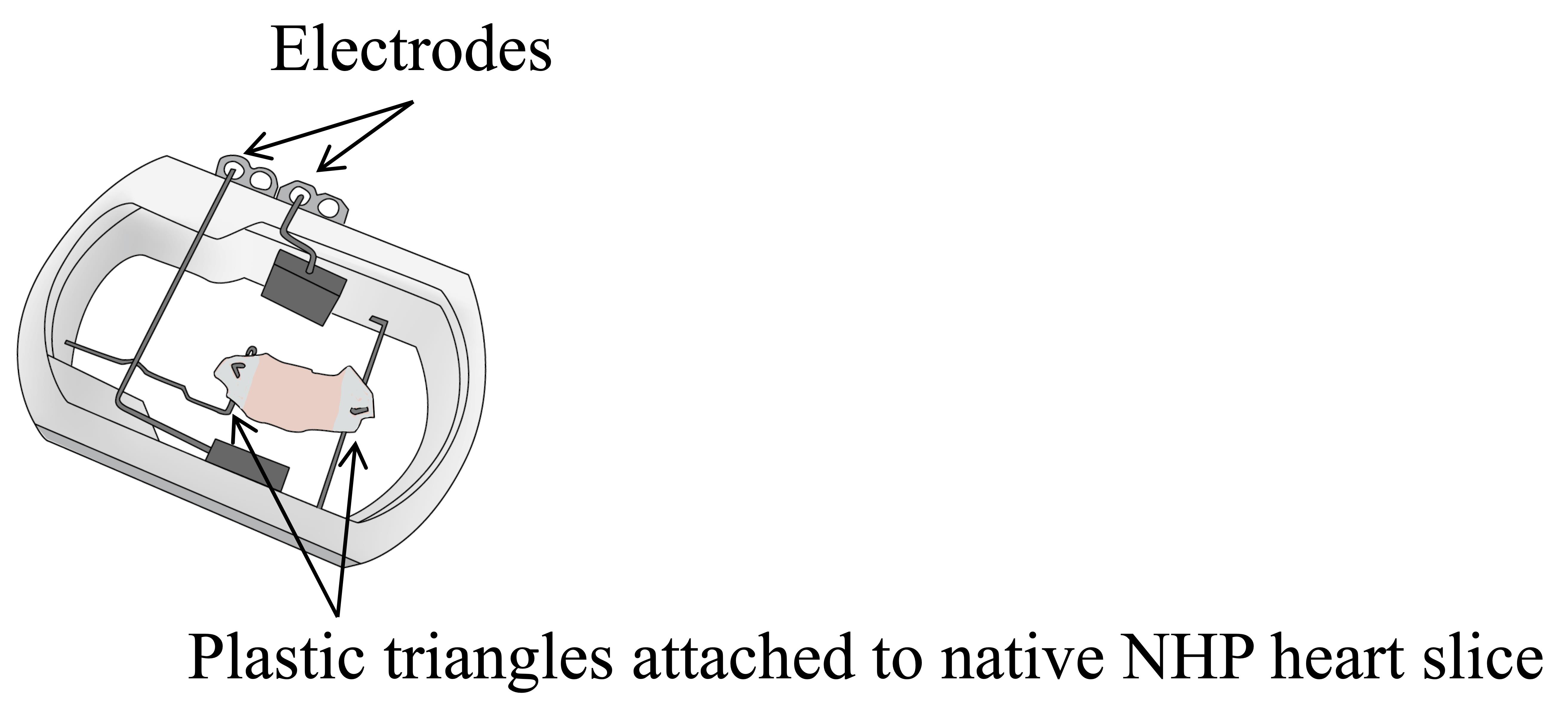
Figure 1. Schematic of assembled MyoDish culture chambers. Electrodes for field stimulation and plastic triangles attached to myocardial slice for anchorage onto spring and holding wires. NHP: non-human primate.
Cutting of tissue slices
Warm M199 culture medium in the water bath (37 °C), add 2.4 mL of medium into each chamber, and place them on the rocker platform in the 37 °C incubator.
Fill 10 mL of 4% agarose into a syringe capped with a Luer lock cap and place it into the water bath until equilibrated to 37 °C.
Turn on the vibratome (placed under laminar flow hood) and reduce Z-distortion of the razor blade using the Vibrocheck device. Sterilize with isopropyl alcohol all parts that may possibly come in contact with the tissue or cutting solution.
The freshly explanted NHP heart needs to be processed immediately after explantation. Cut out a mid-ventricular, approximately 2 cm × 3 cm transmural muscle piece.
Note: Fiber orientation is not ideal if the tissue is excised too apically (make sure to cut mid-ventricular; cutting plane indicated on Figure 2A).
If heart samples cannot be cut and taken into culture immediately (e.g., tissue needs to be shipped), store mid-ventricular samples in a 50 mL capped Falcon tube filled with cutting buffer on wet ice. Delivery should be on the same day.
Place transmural NHP left ventricular tissue blocks into a 35 mm Petri dish containing cold cutting buffer.
Note: The tissue must be continuously stored in cold cutting buffer (4 °C) to avoid hypercontraction and to keep the tissue alive.
Remove endocardial and trabecular layers using sterilized scissors or scalpel. Choose a region without fibrous tissue and of vital color. Orient the tissue according to morphology (uniform fiber orientation, vital color) and trim edges with a razor blade to obtain a tissue block of approximately 1.5 cm × 1 cm in size (Figure 2B and 2C). Fiber orientation is best visible at tissue edges; if it is not visible by eye, use a light microscope equipped with a 10× or 20× lens.
Transfer freshly trimmed tissue blocks into a dry 35 mm Petri dish with the epicardium facing down; the bottom should be dry and stick to the dish.
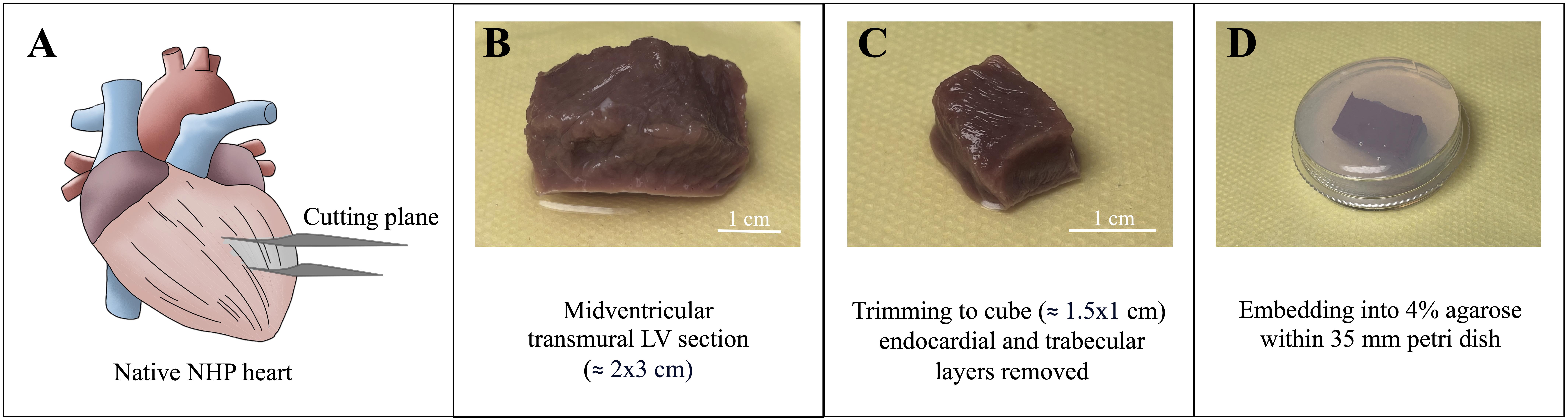
Figure 2. Processing of non-human primate (NHP) heart. A) Schematic of native NHP heart with indicated, mid-ventricular cutting plane on the left ventricle. B) Mid-ventricular transmural left ventricular (LV) section of ~2 cm × 3 cm. Epicardium facing down and endocardial and trabecular layers facing upward. C) Trimmed LV cube (~1.5 cm × 1 cm) after removal of endocardial and trabecular layers. Uniform, diagonal fiber orientation is visible. Epicardium facing down. D) Trimmed tissue block embedded in 4% agarose within 35 mm Petri dish. Epicardium facing down.Embed tissue pieces in preheated (37 °C) 4% agarose (up to two pieces in one 35 mm dish) and let solidify for approximately 3–5 min (Figure 2D). Remove excessive agarose and trim to rectangular shape.
Note: Leave a minimum of approximately 5 mm of agarose around the tissue to ensure stability during cutting process.
Attach the prepared tissue-agarose blocks to the vibratome cutting tray (epicardium facing down; Figure 3) by using histoacryl tissue glue.
Note: Make sure that all edges are well attached to the surface.
Place the vibratome cutting tray into the cutting bath filled with 4 °C cutting buffer. Tissue edges should be parallel to the razor blade (Figure 3).
Place wet ice into the outside tub of the vibratome (ice bath) to make sure that the tissue is continuously cooled to 4 °C during the cutting process (Figure 3).
Move the razor blade towards the tissue, set the borders, and start cutting with a vibration amplitude of 1.3 mm at a speed of 0.07 mm/s and thickness of 300 μm.
Note: Do not increase the cutting speed once the razor blade reaches the tissue. Discard the first slices in which the tissue is not evenly cut.
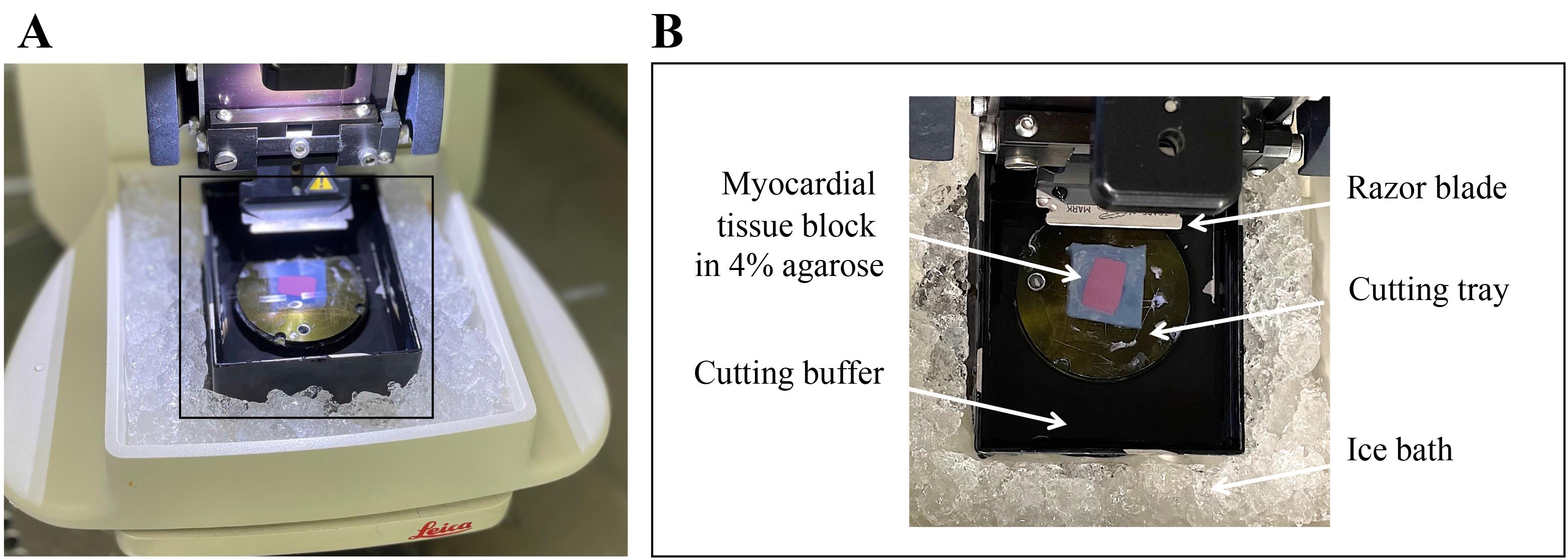
Figure 3. Image of vibratome cutting. A) Overview of vibratome prepared for cutting. B) Zoom into boxed area showing vibratome bath filled with cutting buffer surrounded by an ice bath (filled with wet ice). Myocardial tissue block in 4% agarose glued to vibratome cutting tray. Razor blade adjusted parallel to tissue block edges.Transfer one slice from the vibratome bath to the lid of a Petri dish using tweezers. Detach the slice from agarose with tweezers and make sure to only touch the tissue at the borders.
Remove any residual cutting buffer from the surface with a pipette (do not touch the tissue directly) to prepare the slice to be attached to plastic triangles.
Pipette a stripe of approximately 10 μL of histoacryl glue to the surface of a Petri dish, dip the edge of a plastic triangle into it, and quickly press it onto the edge of the tissue slice. Make sure to attach the triangle perpendicular to the orientation of the muscle fibers (Figure 4A and 4B). If fiber orientation is not visible by eye, use a light microscope for magnification (Figure 4C).
Attach the second plastic triangle in a similar manner on the opposite site of the tissue. Afterwards, trim the tissue on the edges to the size of the triangle.
Repeat the procedure with the other freshly cut tissue slices in the vibratome bath. Make sure to always glue the triangles according to fiber direction.
Note: Slices with attached plastic triangles can be stored in cold cutting buffer up to 1 h before moving them to culture dishes.
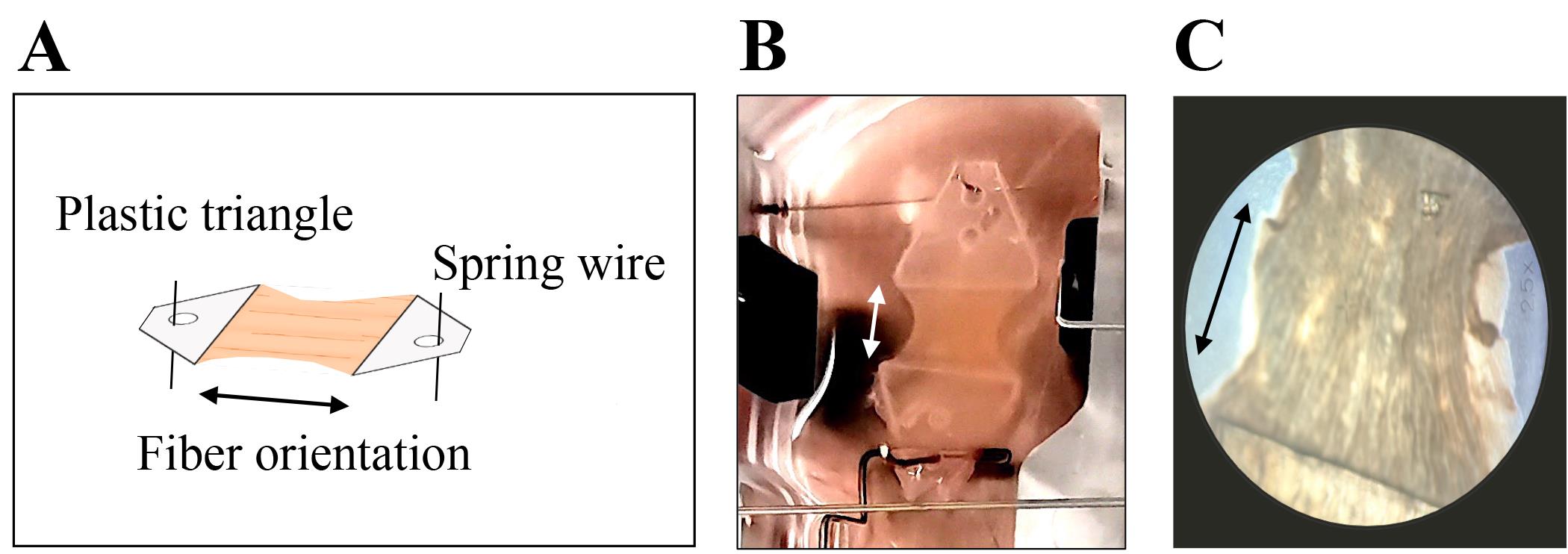
Figure 4. Myocardial slice with attached plastic triangles rectangular to muscle fiber orientation. A) schematic and B) representative image of myocardial slice within MyoDish culture chamber with C) 25-fold magnification to visualize fiber orientation. Arrows indicate fiber orientation.Note: Plastic triangles are equipped with a pinhole to be anchored on spring and holding wires.
Transfer to biomimetic culture chambers
Take an assembled MyoDish culture chamber containing 2.4 mL of M199 medium from the incubator. Hook the slices onto the spring and holding wires with tweezers. Start with the attachment to the upper spring wire. The second triangle is pinned onto the lower holding wire after adjustment of the correct distance according to the individual length of the tissue (Figure 5).
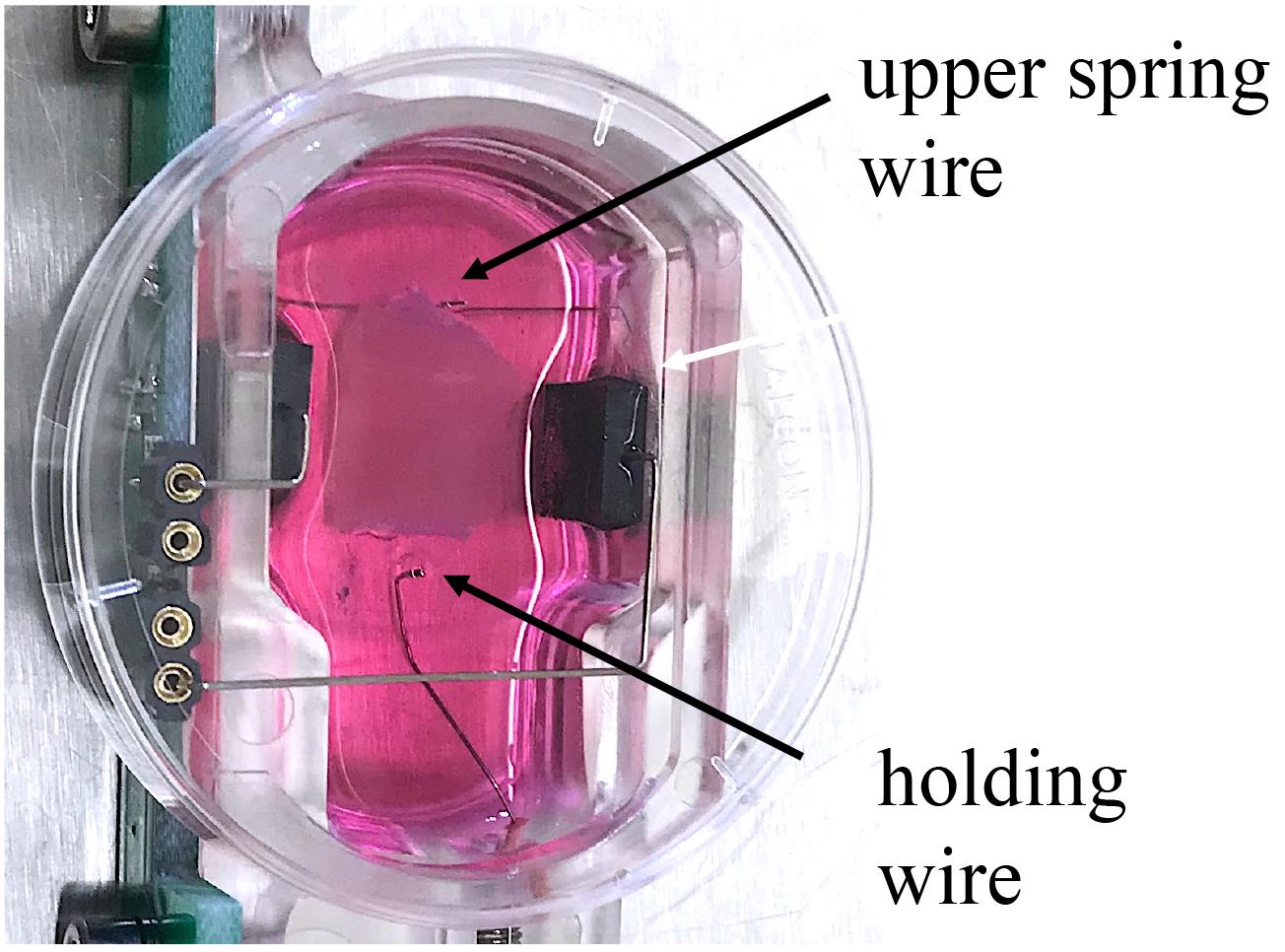
Figure 5. Image of biomimetic MyoDish culture chamber with 2.4 mL of M199 medium. The non-human primate (NHP) myocardial slice is pinned on the upper spring and holding wires before adjusting the preload.Submerge the slice into the medium and stretch the tissue until it is properly aligned.
Cover the chamber with a lid and transfer it to the incubator. Make sure to have a constant temperature of 37 °C in the incubator, as temperature changes can influence viability.
Carefully increase the diastolic tension by increasing the tissue stretch until a mere deflection of contraction is detectable (= baseline).
Adjust the diastolic force to a physiologic preload of 1 mN by progressively increasing the tissue stretch from baseline to 1,000 units. Sharp contraction signals should be detectable immediately. This adjustment of the preload should be repeated after the first 48 h in culture.
Start electrical stimulation with 50 mA at a frequency of 1 Hz.
Start rocking of slices with a target frequency of 60 rpm.
Start data recording by entering the storage location and file name and choose autosave settings (set it to 24 h). Detailed information on technical settings can be found on the manufacturer’s homepage (www.invitrosys.com).
Medium change
Perform medium change every other day by leaving a residual volume of 0.6 mL of old medium in the dish followed by addition of 1.8 mL of fresh, prewarmed M199 medium. Ensure that the fresh medium remains heated to 37 °C during the whole medium exchange procedure.
Exchange medium in all culture chambers one after the other and make sure you place the chamber immediately back into the incubator. Ensure that the atmosphere and temperature in the incubator are not changing significantly during this process, as this is a critical step for successful long-term cultures.
Data analysis
Save files with raw data of measurements in one folder.
Start MyoDish Data File Converter v1.1 and convert files into Axon binary format (.abf).
Select LabChart Reader as the default program for .abf files and open .abf files.
LabChart Reader displays all selected channels among each other in different colors. Set all channels into “autoscale” (command in top bar).
Adjust the timeline displayed at the bottom right (how many measurements per screen pixel). Adjust the timescale by clicking right on the time axis (e.g., display in seconds from start of the file). In the display settings (Setup menu) deselect “Lines between blocks.” This way, a full view of contractile force during the whole culture period, as well as analysis of single days, is possible (Figure 6).
Measure the contraction force by determination of the amplitude. Define one column of the Data Pad as “cyclic measurements” and assign it to the calculation of “average cyclic height.” Choose “peak detection” in the setup panel of the column. Mark a registration period of interest in the data tracing and choose “Add to Data Pad.” The average value of all beat amplitudes within this time period will be displayed in the most recent line of the Data Pad.
Copy the Data Pad to a spreadsheet program (e.g., Microsoft Excel) and calculate contraction force by dividing the arbitrary units by the calibration value supplied with the specific chamber (approximately 1,000 AU/mN).
Export the table of contractile force in mN of any desired days (e.g., to GraphPad Prism) for statistical analysis. A minimum of six patches is usually chosen for statistical comparison using repeated measures ANOVA.
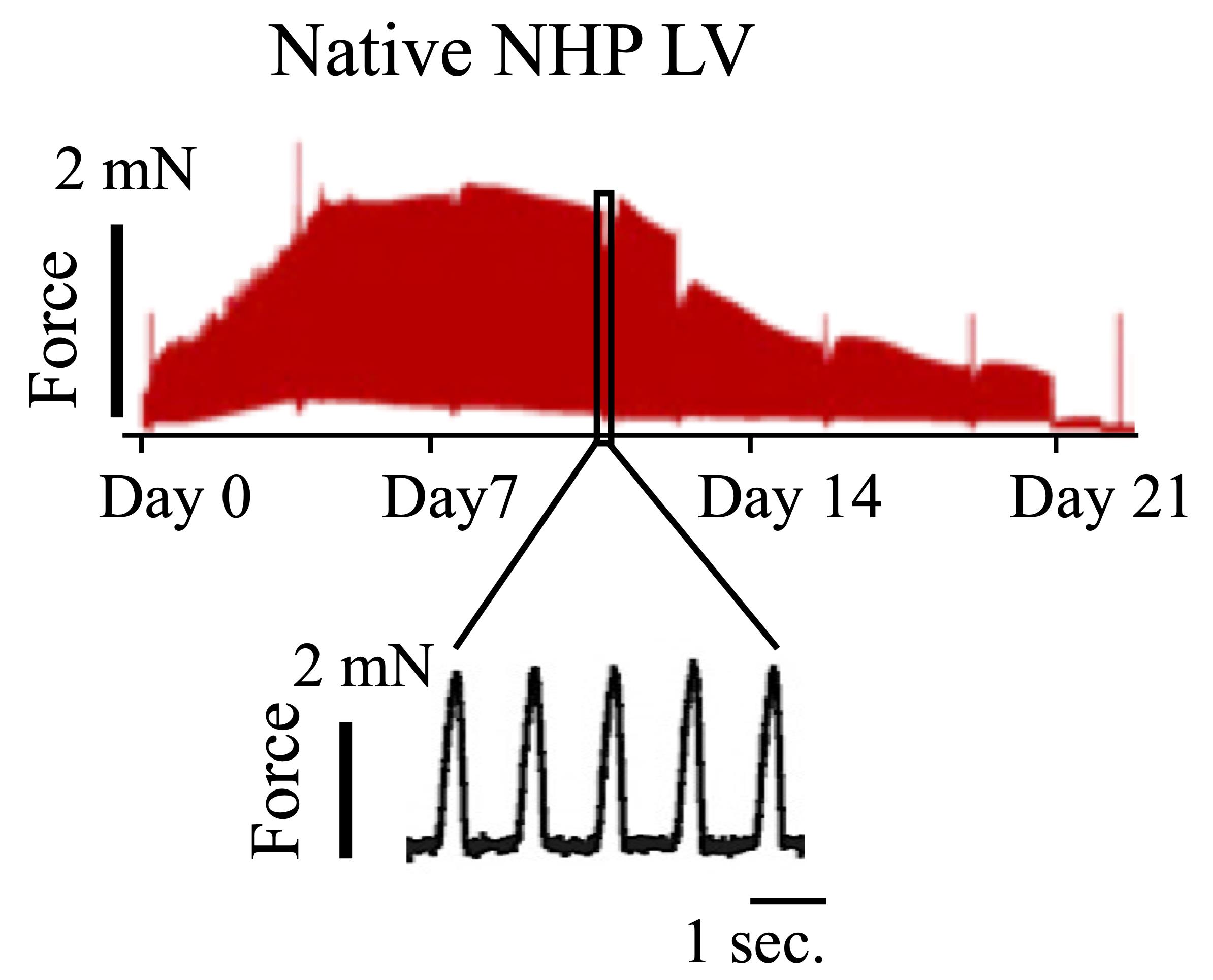
Figure 6. Representative contractile force trace of native non-human primate (NHP) heart slice. Readout of the entire culture period (top) or certain timepoints (bottom) can be generated with LabChart Reader (adapted from Poch et al., 2022).
Recipes
Cutting buffer
pH 7.4, adjust with 1 M NaOH. Filter and store up to three months at 4 °C.
Reagent Concentration (g/L) mM NaCl
KCl
MgCl2
NaH2PO4
Glucose
CaCl2
BDM (2,3-Butanedione)
HEPES
8
0.4
0.2
0.046
2
0.13
3
1.2
136
5.4
1
0.33
10
0.9
30
5
4% agarose preparation
Dissolve agarose at 80 °C for 15 min. Store up to three months at 4 °C. Add 625 μL of HCl for 1 L.
Reagent Concentration (g/L) mM NaCl
KCl
MgCl2
NaH2PO4
CaCl2
BDM (2,3-Butanedione)
HEPES
8
0.4
0.2
0.046
0.13
3
1.2
136
5.4
1
0.33
0.9
30
5
Culture medium M199
50 mL for 16 slices. Filter, store up to one week at 4 °C.
Reagent Amount M199
ITS
β-Mercaptoethanol (14 M)49 mL
500 μL (1:100)
50 μM; mix 1.8 μL of 14M β-Mercaptoethanol stock solution with 0.5 mL of medium. Add 50 μL (= 1:1,000) to M199 culture medium.
Acknowledgments
This work was supported by grants from: European Research Council (ERC) 788381 (to A.M.); the German Research Foundation, Transregio Research Unit 152 (to A.M., K.-L.L.) and 267 (to A.M., K.-L.L.) and DZHK (German Centre for Cardiovascular Research).
We acknowledge the original research papers that this protocol was derived from Poch et al. (2022) and Fischer et al. (2019).
Competing interests
A.D. holds a patent on the technology of biomimetic cultivation and is co-founder and shareholder of InVitroSys GmbH. The remaining authors declare no competing interests.
References
- Brandenburger, M., Wenzel, J., Bogdan, R., Richardt, D., Nguemo, F., Reppel, M., Hescheler, J., Terlau, H. and Dendorfer, A. (2012). Organotypic slice culture from human adult ventricular myocardium. Cardiovasc Res 93(1): 50-59.
- Cox, L. A., Olivier, M., Spradling-Reeves, K., Karere, G. M., Comuzzie, A. G. and VandeBerg, J. L. (2017). Nonhuman Primates and Translational Research-Cardiovascular Disease. ILAR J 58(2): 235-250.
- Fischer, C., Milting, H., Fein, E., Reiser, E., Lu, K., Seidel, T., Schinner, C., Schwarzmayr, T., Schramm, R., Tomasi, R., et al. (2019). Long-term functional and structural preservation of precision-cut human myocardium under continuous electromechanical stimulation in vitro. Nat Commun 10(1): 117.
- Liu, H., Bersell, K. and Kühn, B. (2021). Isolation and Characterization of Intact Cardiomyocytes from Frozen and Fresh Human Myocardium and Mouse Hearts. Methods Mol Biol 2158: 199-210.
- Perbellini, F., Watson, S. A., Scigliano, M., Alayoubi, S., Tkach, S., Bardi, I., Quaife, N., Kane, C., Dufton, N. P., Simon, A., et al. (2018). Investigation of cardiac fibroblasts using myocardial slices. Cardiovasc Res 114(1): 77-89.
- Poch, C. M., Foo, K. S., De Angelis, M. T., Jennbacken, K., Santamaria, G., Bahr, A., Wang, Q. D., Reiter, F., Hornaschewitz, N., Zawada, D., et al. (2022). Migratory and anti-fibrotic programmes define the regenerative potential of human cardiac progenitors. Nat Cell Biol 24(5): 659-671.
- Qiao, Y., Dong, Q., Li, B., Obaid, S., Miccile, C., Yin, R. T., Talapatra, T., Lin, Z., Li, S., Li, Z., et al. (2019). Multiparametric slice culture platform for the investigation of human cardiac tissue physiology. Prog Biophys Mol Biol 144: 139-150.
- Watson, S. A., Scigliano, M., Bardi, I., Ascione, R., Terracciano, C. M. and Perbellini, F. (2017). Preparation of viable adult ventricular myocardial slices from large and small mammals. Nat Protoc 12(12): 2623-2639.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Poch, C. M., Dendorfer, A., Laugwitz, K. L. and Moretti, A. (2023). Ex vivo Culture and Contractile Force Measurements of Non-human Primate Heart Slices. Bio-protocol 13(13): e4750. DOI: 10.21769/BioProtoc.4750.
Category
Medicine > Cardiovascular system > Heart tissue culture techniques
Microbiology > Microbe-host interactions > Ex vivo model
Drug Discovery > Drug Screening > Anti-infective agents
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









