- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Simple and Reproducible Stereomicroscopic Method to Assess Fungal Biofilms: Application to Antifungal Susceptibility Testing
Published: Vol 13, Iss 13, Jul 5, 2023 DOI: 10.21769/BioProtoc.4713 Views: 1557
Reviewed by: Wenrong HeYe XuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Cryptococcus neoformans Virulence Assay Using a Galleria mellonella Larvae Model System
Piotr R. Stempinski [...] Arturo Casadevall
Aug 5, 2022 2998 Views
Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
Piao Yang [...] Ye Xia
Oct 20, 2023 2639 Views
In Vitro Hyphal Branching Assay Using Rhizophagus irregularis
Takaya Tominaga and Hironori Kaminaka
Aug 20, 2024 2231 Views
Abstract
Candida albicans, a well-known opportunistic pathogen, is a major cause of human fungal infections. Biofilm formation is considered an important pathogenesis factor. Biofilms are less sensitive to antibiotics and immune responses, allowing them to colonize and persist in host niches. Biofilm screening is important in the identification of anti-biofilm drugs. However, developing nations, with limited financial resources, often do not have access to advanced scientific equipment. Here, we describe an in vitro, protocol using common materials and simple equipment to evaluate static microbial biofilms.
Keywords: Antimicrobial resistanceBackground
Candida albicans, a commensal fungus, is a leading cause of human fungal infections. It becomes a very resilient pathogen under low host immunity, for example in neonates or patients with AIDS or transplants (Ostrosky-Zeichner et al., 2003). Biofilm formed by this pathogen plays important roles in both its virulence and antifungal resistance (Gulati and Nobile, 2016). Various microscopic methods are routinely used to evaluate Candida, including microscopic (atomic force, epifluorescence, laser scanning confocal, and scanning electron) assays. The simple technique described herein is more cost effective, in particular for biofilm screening projects.
The most common method to evaluate biofilm formation is the microplate assay (Azeredo et al., 2017). Here, we present a low-cost visual biofilm detection protocol that uses tissue culture plates and detection in brightfield that offers comparable results to standard assays (Shahina et al., 2022a and 2022b). This assay (Figure 1) is suitable for researchers in developing countries where there is a lack of access to high throughput microscopy and microplate readers.
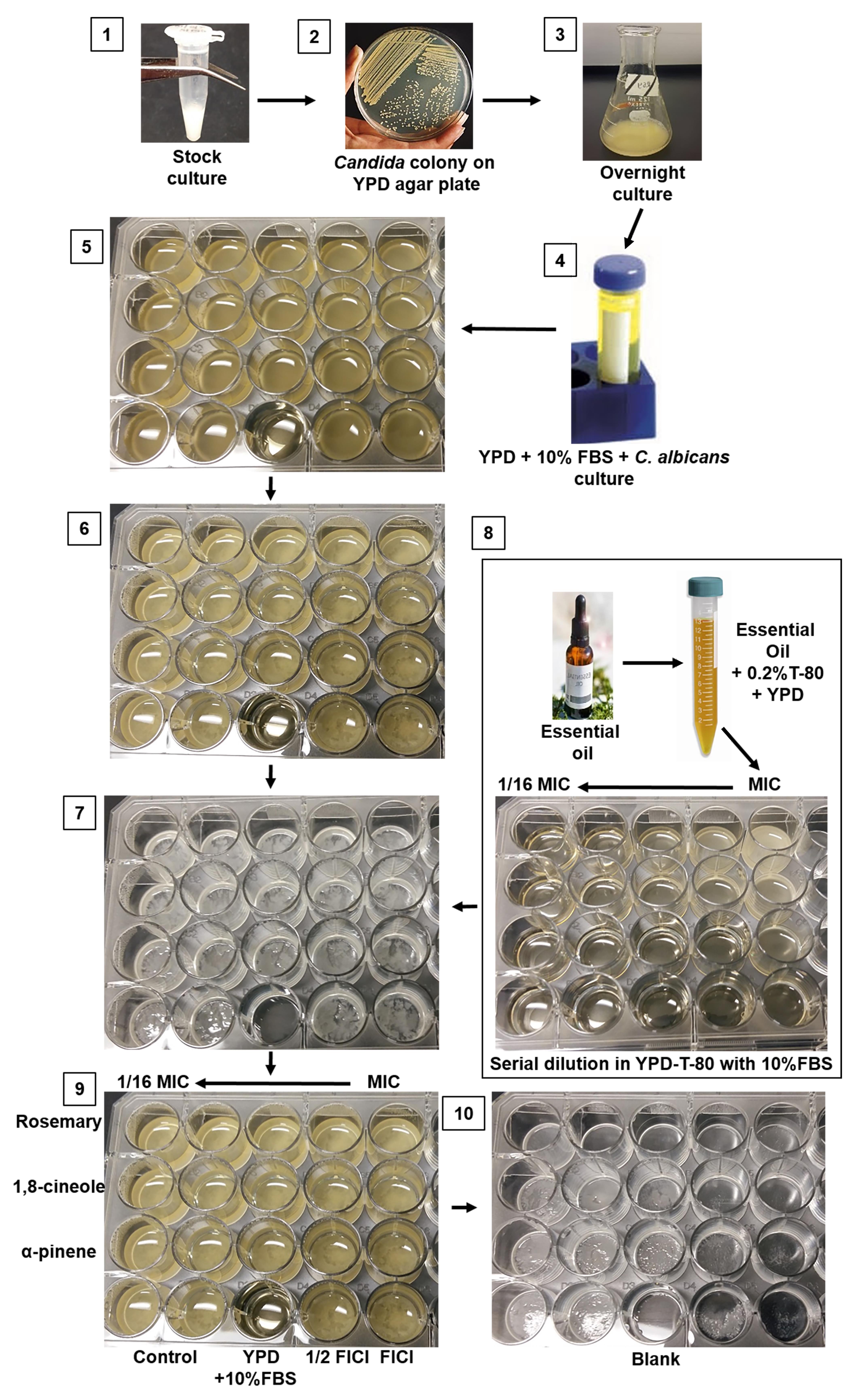
Figure 1. Schematic step-by-step protocol for visualizing C. albicans biofilm formation and inhibition. 1. C. albicans stock culture in 50% glycerol. 2. Colony formation on YPD media. 3. Overnight culture on YPD broth. 4. Sample in prewarmed 10% FBS with YPD diluted according to desired cell density. 5. Candida in 24-well plate (plate 1) in preparation for incubation at 37 °C for 24 h. 6. Sample plate after 24 h incubation. 7. Biofilm growth at the bottom of the 24-well plate following removal of the medium and planktonic cells. 8. Preparation of drugs/essential oils in a new plate (plate 2) and its transfer into the pre-formed biofilm (plate 1). 9. Biofilm with antifungal drugs (plate 1) is ready for incubation at 37 °C for 24 h. 10. After 24 h incubation (plate 1), the media is aspirated, and the plate is ready for stereomicroscopic imaging.
Materials and reagents
15 mL screw cap conical bottom tube (Sarstedt, catalog number: 50-809-221)
24-well tissue culture plates (Sarstedt, catalog number: 83.3922)
50 mL screw cap conical bottom tube (Sarstedt, catalog number: 50-809-218)
96-well, flat base, PS, transparent plate (Sarstedt, catalog number: 82.1581001)
Syringe filter, Filtropur S, 0.2 μm (Sarstedt, catalog number: 83.1826.001)
Test tube racks (Fisher Scientific, catalog number: 14-809-62) or any standard test tube racks
Agar (VWR, catalog number: 97064-336)
Antifungal agent; amphotericin B (Sigma-Aldrich, catalog number: 1397-89-3)
BactoTM peptone (Becton Dickinson, catalog number: 211677)
D-(+)-Glucose (Sigma-Aldrich, catalog number: G8270)
Essential oils:
RM (Aroma force, catalog number: 6658117001)
1,8-cineole, 99% (Acros organics, catalog number: 470-82-6)
(+)–α-pinene ≥ 99% (Sigma-Aldrich, catalog number: 268070)
Fetal bovine serum (FBS) (Gibco, catalog number: 12483-020)
Parafilm (Amcor, catalog number: PM996)
Yeast extract (Sigma-Aldrich, catalog number: 70161)
TWEEN® 80, polysorbate 80 (polyoxyethylene sorbitan monooleate) (Sigma-Aldrich, catalog number: 9005-65-6)
Yeast extract peptone (YPD) plate containing freshly revive C. albicans colonies
YPD agar medium (per liter) (see Recipes)
YPD broth (per liter) (see Recipes)
YPD broth containing 10% FBS (see Recipes)
Equipment
-20 °C freezer (General Electrics)
-80 °C freezer (Revco, catalog number: ULT2586-4-A)
Autoclave (Steris Scientific, AMSCO® C series small steam sterilizer) or any standard autoclave
Balance (Mettler Toledo NewClassic MF Precision Balance, catalog number: ML303E/03)
Benchtop shaking incubator (CorningTM LSETM, catalog number: CLS6791)
Inoculating loop (FisherbrandTM, catalog number: 131045)
Laminar flow workstation (MicroZone Corporation, model: V4-MN-99-030) or any standard equipment with similar features
Microbiological benchtop laboratory incubator (Thelco, Model 4)
Micropipette 100–1,000 μL (Eppendorf, catalog number: 3123000063)
Micropipette 20–200 μL (Eppendorf, catalog number: 3123000055)
Micropipette 100–1,000 μL tips (VWR International, catalog number: 83007-376)
Micropipette 20–200 μL tips (Sarstedt, catalog number: 70.3030)
Micropipette 500–5,000 μL tips (Sarstedt, catalog number: 70.1183.002)
Petri dish, 92 mm × 16 mm (Sarstedt, catalog number: 82.1473.001)
Pipette, 500–5,000 μL (Eppendorf, catalog number: 3123000071)
Plate reader for OD determination (BioTek Instruments, Epoch- Microplate Spectrophotometer)
Note: The plate reader was used for verification of this method; a standard spectrophotometer (supplier model T, A, C. Detector: Silicon Photodiode or Thermo ScientificTM GENESYSTM Visible and UV-Visible Spectrophotometers) can be used to measure OD.
PYREX glass erlenmeyer flask, 125 mL (Corning, catalog number: 4980-125)
Stereomicroscope (Nikon’s SMZ 1500) equipped with a 2× and 4× objective and a digital camera or eyepieces for use of a mobile phone camera
Procedure
Candida growth
Use a sterile inoculating loop to remove culture from the glycerol stock culture (-80 °C) and inoculate a YPD plate (Table 1, Day 1).
Table 1. Protocol steps
Total time Workflow Condition Day 1 Streak C. albicans on YPD plate Incubate for 24 h at 30 °C in the standby incubator Day 2 Inoculate a small, round colony into 10 mL of YPD broth Incubate for 14–16 h at 30 °C until mid-logarithmic phase in a shaking incubator at 200 rpm Day 3 Prepare 24-well plate with culture for biofilm growth Incubate for 24 h at 37 °C in a shaker at 75 rpm Day 4 After removing the non-adherent cells, add antifungal agents for biofilm inhibition experiments Again, incubate for 24 h at 37 °C in a shaker at 75 rpm
Note: Low shaking speed reduces the chance of biofilm detachment from the bottom surface of the plate.
Day 5 Assess biofilm by imaging in brightfield Incubate the plate at 30 °C for 24–36 h.
Inoculate a single colony from the cultured plate into a sterile 100 mL conical flask containing 10 mL of YPD broth (Table 1, Day 2).
Incubate the flask at 30 °C for 16 h with constant shaking at 200 rpm (Figure 1).
Biofilm formation
Measure the OD600 of the overnight Candida culture and dilute in YPD medium supplemented with 10% FBS to a starting concentration of 107 CFU/mL (Table 1, Day 3).
Note: Use a standard CFU method (Miller, 1972) or direct microscopic count using Petroff-Hausser counting chambers.
Distribute 500 μL of the Candida suspension to each well of a 24-well tissue culture plate (plate 1) and incubate at 37 °C for 24 h without shaking. For the blank, add 500 μL of YPD medium supplemented with 10% FBS.
Note: For static biofilms, the plate must remain stationary.
The next day, gently remove the media and non-adherent cells from each well (Figure 1).
Note: Tilting the 24-well plates and gently placing the pipette tip in the corner of the well helps to prevent disruption of the fragile biofilm.
Biofilm inhibition assay
Prepare working solutions of antimicrobials (either antifungal drugs or essential oils) at minimum inhibitory concentration (MIC) with proper solubilizing agents (0.2% Tween-80 is suitable for essential oils).
In a new 24-well plate (plate 2), add 500 μL of YPD medium supplemented with 10% FBS to every well except the first. To the first well, add 1,000 μL of antimicrobial working solution (from C, step 1), followed by a serial dilution (in YPD medium supplemented with 10% FBS) to lower concentrations (e.g., 1/16 MIC) along the rows of the plate (Table 1, Day 4).
Note: Washable test tubes (less expensive) can be used to serially dilute the antifungal drugs.
Transfer drugs/plant-based essential oils (EOs) to the plate containing the pre-formed biofilm (plate 2 from step B), including a growth control and blank.
If working with volatile antifungals (e.g., EOs), cover the plate with parafilm to avoid evaporation and incubate the plate at 37 °C for 24 h in the incubator without shaking (Figure 1).
Biofilm visualization
After 24 h, gently aspirate and discard the media from each well (Table 1, Day 5).
Image samples at 4× with a stereomicroscope and capture images either with a digital camera or through the eyepiece using a cellphone camera. Representative images of C. albicans RSY150 biofilms exposed to various essential oil components are shown in Figure 2.
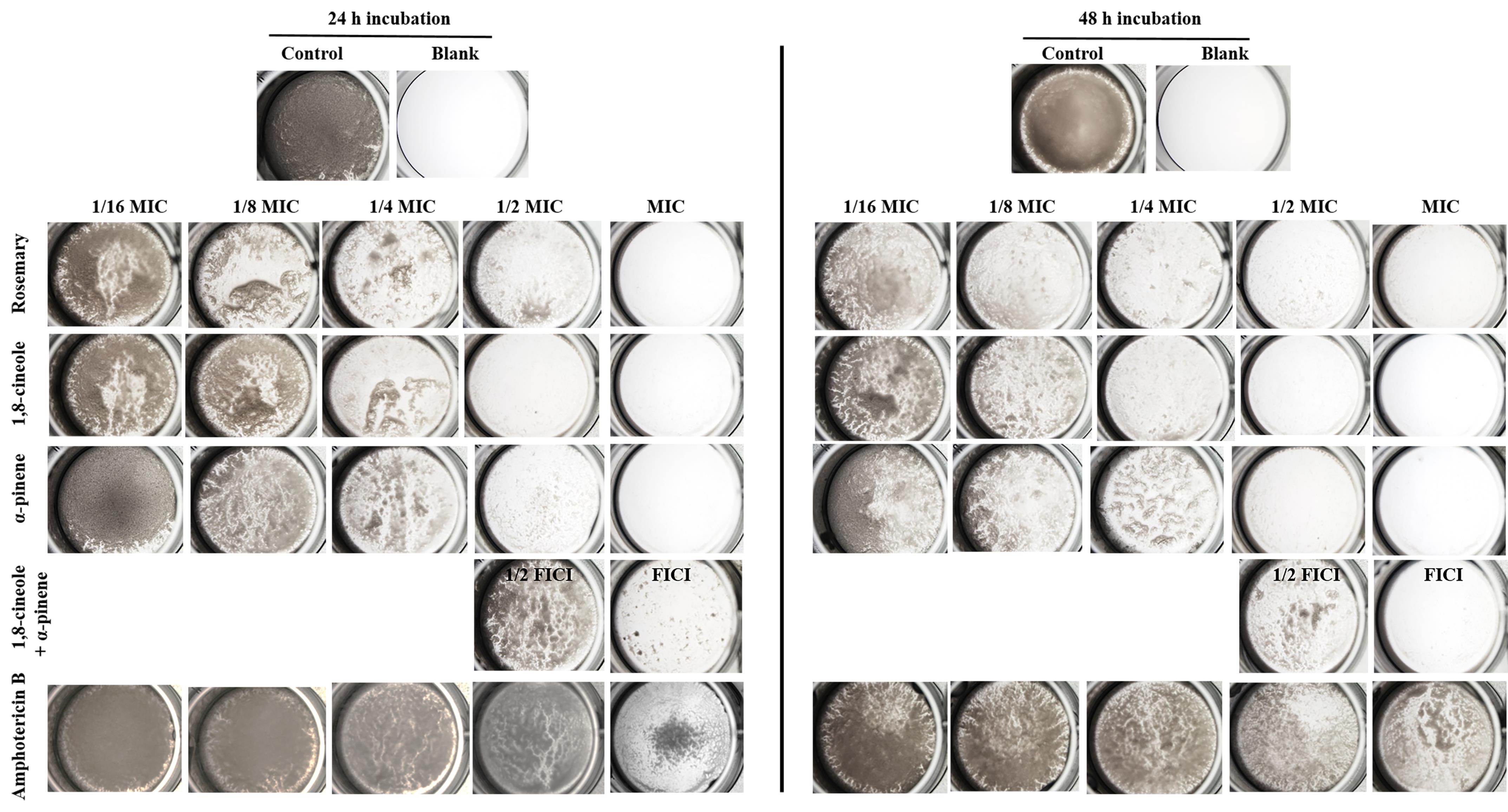
Figure 2. Stereoscopic brightfield images show visual differences between C. albicans RSY150 biofilm mass following exposure to rosemary essential oil, its components 1,8-cineole and α-pinene, Amp B (1/16 MIC to MIC), and the two components at 1/2 and 1 fractional inhibitory concentration index (FICI), compared to control cells. Plates were imaged at 4× with the digital camera mounted on the stereomicroscope.Note: Assessment of biofilm formation and inhibition require experiments in triplicate, including controls, as there can be high variability in biofilm growth. Quantitative assays would require a microplate assay (Crystal violate/MTT/XTT). Many studies suggest 48 h incubation as optimal for biofilm studies (Chandra et al., 2001; Cruz et al., 2018; Ripolles-Avila et al., 2018; Cendra et al., 2019; Galdiero et al., 2020), but 24 h incubation does not require replacement of media as a result of nutrient depletion (Khelissa et al., 2017). FIC estimates the interaction between two or more drugs intended to be used in combination, and the FICI is calculated as the sum of FICs for compounds 1 and 2.
Data analysis
Following this experimental protocol, images from three independent biological replicates (see Figure 2) were used to determine the presence or absence of biofilm, compared with a blank well consisting of equal volumes of experimental media lacking Candida (Shahina et al., 2022a and 2022b).
Recipes
YPD agar medium (per liter)
Glucose 20 g in 1 L flask
Agar 18 g
Yeast extract 10 g
Peptone 20 g
dH2O 1 L
Add all of the above to a 1 L flask, except glucose. Add 800 mL of dH2O and allow the powder to dissolve with gentle stirring.
Autoclave for 40 min at 15 psi at 121 °C.
In the meantime, add 20 g of glucose to 200 mL of dH2O, use a magnetic stirrer to mix well, and sterilize by filtration.
After autoclaving the flasks, allow them to cool to 45 °C, add the sterile-filtered dextrose, and mix well.
Dispense 15 mL of the solution from step d into Petri plates inside a sterile hood and let cool to room temperature.
Note: If either glucose or dextrose are added to the YPD broth powder prior to autoclaving, the sugar will be caramelized, observed as a darker media color. It is good practice to add dextrose separately after the autoclave step once it has been dissolved in water and sterile filtered.
YPD broth (per liter)
Glucose 20 g in 1 L flask
Yeast extract 10 g
Peptone 20 g
dH2O 1 L
Follow the same procedure as described in Recipe 1, but without pouring into the Petri plates.
YPD broth containing 10% FBS
Thaw and warm up 100% FBS, stored as appropriately sized aliquots in the freezer.
Add 50 mL of 100% FBS to 450 mL of YPD broth to obtain 10% FBS in YPD.
Note: Serum is very expensive; therefore, always aliquot and freeze the serum (-80 °C) and add it to the medium just prior to its use. Store unused portions of thawed aliquots in the refrigerator (-20 °C), where they can be stored for several weeks.
Acknowledgments
This work was supported by the National Science and Engineering Research Council (NSERC) grant to TESD (228206-07). Z.S. was partially supported by the Faculty of Graduate Studies and Research at the University of Regina. The University of Regina main campus is situated on the traditional territories of the nêhiyawak, Anihšināpēk, Nakoda, Dakota, and Lakota peoples, and the homeland of the Métis/Michif Nation. This protocol is based on previous work (Shahina et al., 2022a and 2022b).
Competing interests
The authors declare that they have no competing interests.
References
- Azeredo, J., Azevedo, N. F., Briandet, R., Cerca, N., Coenye, T., Costa, A. R., Desvaux, M., Di Bonaventura, G., Hébraud, M., Jaglic, Z., et al. (2017). Critical review on biofilm methods. Crit Rev Microbiol 43(3): 313-351.
- Cendra, M. D. M., Blanco-Cabra, N., Pedraz, L. and Torrents, E. (2019). Optimal environmental and culture conditions allow the in vitro coexistence of Pseudomonas aeruginosa and Staphylococcus aureus in stable biofilms. Sci Rep 9(1): 16284.
- Chandra, J., Kuhn, D. M., Mukherjee, P. K., Hoyer, L. L., McCormick, T. and Ghannoum, M. A. (2001). Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol 183(18): 5385-5394.
- Cruz, C. D., Shah, S. and Tammela, P. (2018). Defining conditions for biofilm inhibition and eradication assays for Gram-positive clinical reference strains. BMC Microbiol 18(1): 173.
- Galdiero, E., de Alteriis, E., De Natale, A., D’Alterio, A., Siciliano, A., Guida, M., Lombardi, L., Falanga, A. and Galdiero, S. (2020). Eradication of Candida albicans persister cell biofilm by the membranotropic peptide gH625. Sci Rep 10(1): 5780.
- Gulati, M. and Nobile, C. J. (2016). Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect 18(5): 310-321.
- Khelissa, S. O., Jama, C., Abdallah, M., Boukherroub, R., Faille, C. and Chihib, N. E. (2017). Effect of incubation duration, growth temperature, and abiotic surface type on cell surface properties, adhesion and pathogenicity of biofilm-detached Staphylococcus aureus cells. AMB Express 7(1): 191.
- Miller, J. H. (1972). Experiments in molecular genetics. Cold Spring Harbor Laboratory. N.Y.
- Ostrosky-Zeichner, L., Rex, J. H., Pappas, P. G., Hamill, R. J., Larsen, R. A., Horowitz, H. W., Powderly, W. G., Hyslop, N., Kauffman, C. A., Cleary, J., et al. (2003). Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob Agents Chemother 47(10): 3149-3154.
- Ripolles-Avila, C., Hascoët, A. S., Guerrero-Navarro, A. E. and Rodríguez-Jerez, J. J. (2018). Establishment of incubation conditions to optimize the in vitro formation of mature Listeria monocytogenes biofilms on food-contact surfaces. Food Control 92: 240-248.
- Shahina, Z., Al Homsi, R., Price, J. D. W., Whiteway, M., Sultana, T. and Dahms, T. E. S. (2022a). Rosemary essential oil and its components 1,8-cineole and alpha-pinene induce ROS-dependent lethality and ROS-independent virulence inhibition in Candida albicans. PloS One 17(11): e0277097.
- Shahina, Z., Ndlovu, E., Persaud, O., Sultana, T. and Dahms, T. E. S. (2022b). Candida albicans Reactive Oxygen Species (ROS)-Dependent Lethality and ROS-Independent Hyphal and Biofilm Inhibition by Eugenol and Citral. Microbiol Spectr 10(6): e0318322.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Shahina, Z. and Dahms, T. E. S. (2023). A Simple and Reproducible Stereomicroscopic Method to Assess Fungal Biofilms: Application to Antifungal Susceptibility Testing. Bio-protocol 13(13): e4713. DOI: 10.21769/BioProtoc.4713.
Category
Microbiology > Microbial biofilm > Killing assay
Biological Sciences > Biological techniques > Microbiology techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









