- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Alginate Gel Immobilization of Caenorhabditis elegans for Optical Calcium Imaging of Neurons
Published: Vol 13, Iss 12, Jun 20, 2023 DOI: 10.21769/BioProtoc.4697 Views: 2248
Reviewed by: Oneil Girish BhalalaAnand Ramesh PatwardhanMatthias Rieckher

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Examining Cocaine Conditioning Place Preference in Mice
MaryElizabeth J. Simkevich [...] André O. White
Apr 20, 2020 5424 Views
Analysis of Caenorhabditis elegans Aging-related Neurodegeneration in Chemosensory Neurons
Cira Crespo and Roberto Grau
Jul 20, 2022 2592 Views

A Novel Composite Method of Post-stroke Epilepsy Induction
Yiting Guo and Raymond Tak Fai Cheung
Jul 20, 2025 1889 Views
Abstract
A fascinating question in neuroscience is how sensory stimuli evoke calcium dynamics in neurons. Caenorhabditis elegans is one of the most suitable models for optically recording high-throughput calcium spikes at single-cell resolution. However, calcium imaging in C. elegans is challenging due to the difficulties associated with immobilizing the organism. Currently, methods for immobilizing worms include entrapment in a microfluidic channel, anesthesia, or adhesion to a glass slide. We have developed a new method to immobilize worms by trapping them in sodium alginate gel. The sodium alginate solution (5%), polymerized with divalent ions, effectively immobilizes worms in the gel. This technique is especially useful for imaging neuronal calcium dynamics during olfactory stimulation. The highly porous and transparent nature of alginate gel allows the optical recording of cellular calcium oscillations in neurons when briefly exposed to odor stimulation.
Keywords: ImmobilizationBackground
Caenorhabditis elegans is an ideal model organism to observe dynamic changes at the cellular level using fluorescence labeling of target molecules owing to its transparent nature. Unfortunately, worms are sensitive to light and become very active when placed under a microscope. For optical calcium imaging, immobilizing the worms for a prolonged period is essential; however, this can be difficult due to movement artifacts.
Anesthetic agents, such as sodium azide or levamisole, are the most popular choice for immobilizing worms; however, they can cause physiological and behavioral variations in the organism (Fang-Yen et al., 2012; Lewis et al., 1980). As an alternative, gluing worms to a thin agar pad using cyanoacrylate adhesive has been used in calcium imaging and electrophysiological studies (Kerr et al., 2000). This method allows worms to recognize and respond to external stimuli more efficiently; however, it can be challenging to master, particularly gluing the worm only to the tail region. Often, worms become mired when glue sticks to the trunk and head and cannot be used for the experiment (Kerr et al., 2000). Additionally, it is difficult to measure calcium kinetics in the head neurons using this method because of the quick head turns of the worm.
Microfluidic chips have been employed to trap worms for extended kinetic measurements (Hulme et al., 2007). This expensive technique requires the use of precise microfluidic channels and fluid pumps. Polystyrene beads (0.1 μm size) on 5%–10% agarose pads have been used to immobilize worms for extended measurement (Kim et al., 2013). Despite its simplicity, bead-based immobilization triggers dopamine levels in worms (Dong et al., 2018; Raj and Thekkuveettil, 2022) and can compromise the interpretation of the results.
Here, we present a new protocol for immobilizing worms using sodium alginate gel. This method is quick, cost-effective, and causes minimal distress to worms. The gel creates a protective cavity around them, similar to that of microfluidic channels. The alginate gel is highly porous and enables olfactory signals to reach the worm. To prove the efficacy of this method, we applied it to a functional imaging application and showed that it can accurately measure calcium spikes in neurons during odor exposure.
Materials and reagents
Materials
Sodium alginate powder (SDFCL SD Fine Chem Ltd, Mumbai. CAS No. 9005-38-3)
Cover glass, 22 mm × 40 mm (Labtech medico P Ltd, catalog number: LT-71)
Calcium chloride (HiMedia, CAS No. 10035-04-8)
Whatman filter paper, pore size 11 μm (Whatman, catalog number: 1001-917)
Isoamyl alcohol (IAA) (Central Drug House, Bombay, CAS No. 123-51-3)
Equipment
Leica DMi8 automated fluorescent microscope (S/N 455551) with Leica DFC7000 T camera
InjectMan 4 (Eppendorf)
Software
GraphPad Prism 6 software
Las X image acquisition software
Procedure
Preparation of 5% sodium alginate
Dissolve 1 g of sodium alginate in 20 mL sterile distilled water in a 50 mL beaker to make a 5% sodium alginate solution.
Stir the solution overnight using a magnetic stirrer at room temperature until the final solution is clear and bubble-free.
Worm block preparation
On a coverslip, add a small drop of 5% sodium alginate solution at room temperature and slightly spread it to make it sufficiently thick for the worms to immerse.
Place the worms (one can add 1–4 worms per coverslip) in the sodium alginate layer.
Allow the worms to settle for 2 min with minimum thrashing movements. To avoid overflow of the solution, place a plastic ring of 0.5 cm diameter as a barrier. To the inside of the ring, add 100 μL of 100 mM CaCl2 (as the divalent ions) to polymerize the sodium alginate solution with the worm entrapped inside. Within 2–3 min, the sodium alginate will polymerize at room temperature (see Figure 1).
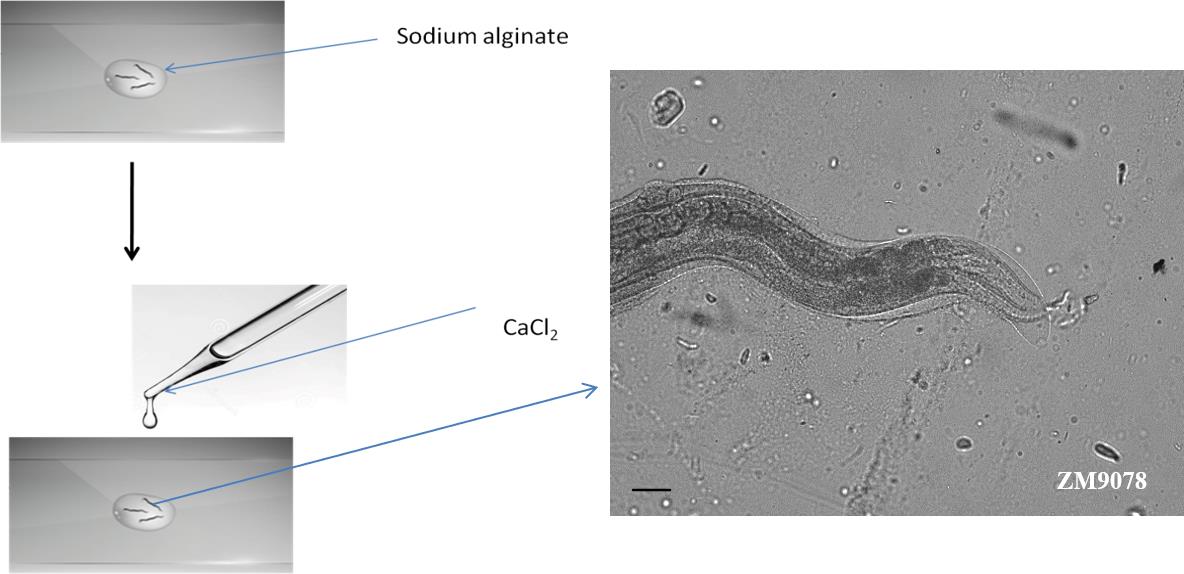
Figure 1. Preparation of alginate scaffold to entrap worms. Worms were placed in the 5% sodium alginate prior to the addition of 100 mM CaCl2 to polymerize sodium alginate solution. Microscopic image showing an immobilized worm.After polymerization, remove the plastic ring and wipe off the excess CaCl2 solution using filter paper. Alginate polymerization will occur around the worm, and the worms will show restricted movements.
Worm viability within alginate gel
Observe the immobilized worms for 2 min to record their movement with and without exposure to fluorescent light.
The head bends can be measured as a sign of viability.
The worms can be kept in the alginate scaffold for more than 30 min at room temperature.
The worms can be kept in a humid chamber to avoid dehydration for repeated recording.
Olfactory stimulation
As the odor delivery system for this study, a piece of Whatman filter paper (0.3 × 0.3 mm) was placed on the tip of a glass capillary tube.
Attach the capillary tube to the Eppendorf InjectMan’s arm in order to measure and maintain a constant distance between the worm and the odor source (Figure 2).
In order to measure the calcium kinetics in ring interneuron D (RID) neurons, stimulate the strain ZM9078 (hpls587–GcaMP6) with 1/300 diluted IAA.
Saturate the Whatman filter paper with 5 μL of the 1/300 diluted IAA and keep it 1 mm away from the worm.
Move the capillary away from the worm using the joystick after 20 s of exposure.

Figure 2. Setup for olfactory stimulation. Leica DMi8 automated fluorescent microscope with the Eppendorf InjectMan. A. Whatman filter paper. B. capillary tube. C. Eppendorf InjectMan’s arm.
Image acquisition
For calcium imaging, use a Leica DMi8 automated fluorescent microscope and LasX image acquisition software to manage the imaging process (Leica).
Use 20× magnification to capture time-lapse pictures.
The exposure time for fluorescence imaging is 150 ms.
Leica DFC7000T camera time-lapse sequences are captured for up to 2 min (2 frames/s).
Image processing
Perform image processing with Fiji software. Capture images in the JPG file format.
Draw a region of interest (ROI) over the RID motor neuron in ZM9078 (hpIs587–GCaMP6) strain.
Two macro files were written for image processing.
ROI_Calulation.ijm
// This macro moves across images
// Finds the maximum pixel value
for (i=1; i<=nImages; i++) {
selectImage(i);
processImage(); //or simply put your processing commands here.
}
function processImage() {
getRawStatistics(nPixels, mean, min, max);
run("Find Maxima...", "noise="+max+" output=[Point Selection]");
getSelectionBounds(x, y, w, h);
makeOval((x-70), (y-40), 140, 90);
}
Measure_Values.ijm
// This macro moves across images
// Finds the maximum pixel value
for (i=1; i<=nImages; i++) {
selectImage(i);
// run("Set Measurements...", "area mean min integrated redirect=None decimal=3");
run("Measure");
}
Use ROI_calculation.ijm macro to draw ROI in the images.
Calculate fluorescent intensity measurement with Measure_values.ijm macro (see Figure 3).
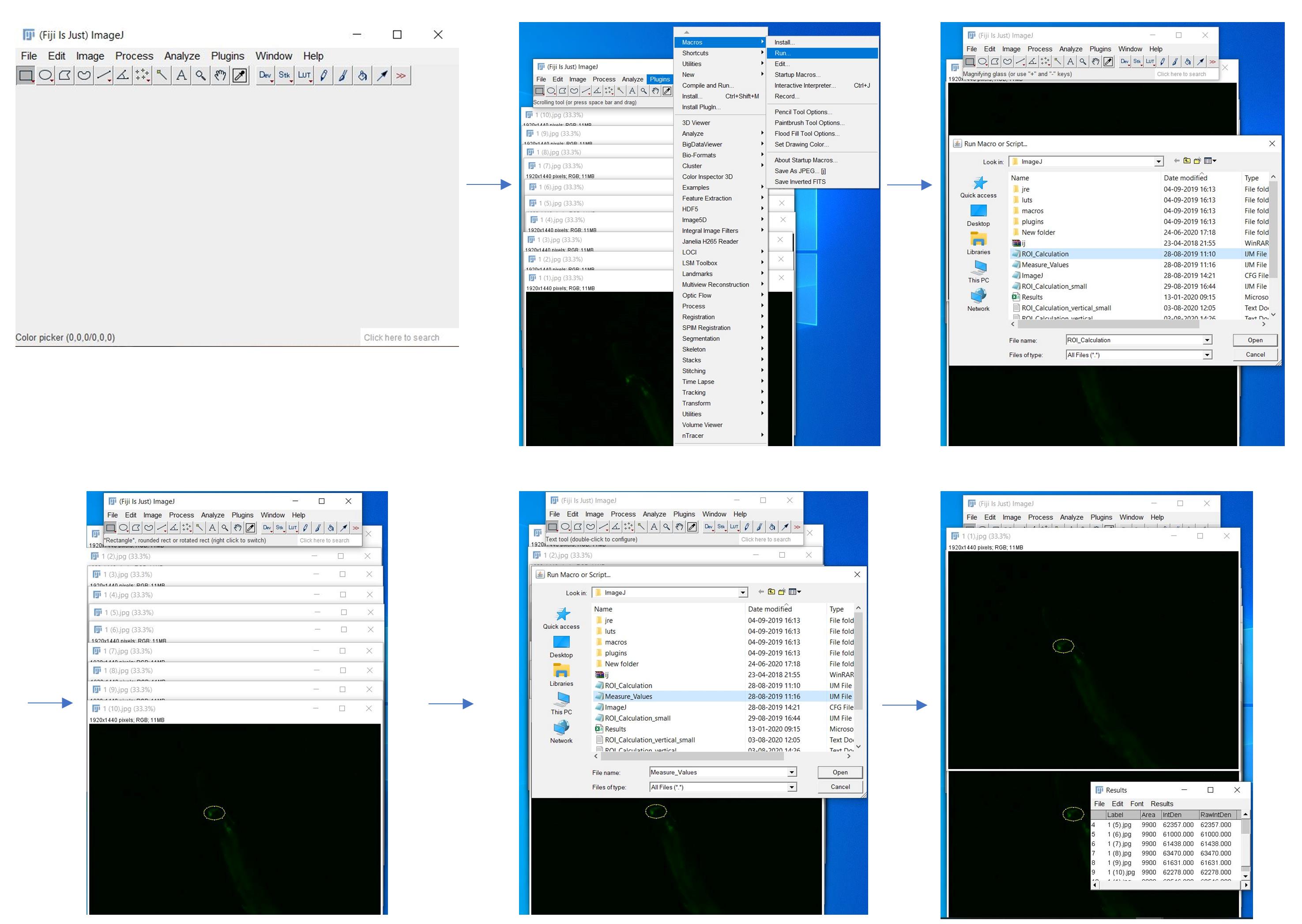
Figure 3. Screenshots of each step of image processing using Fiji softwareCalculate change in fluorescent intensity by subtracting the fluorescent intensity of subsequent images from the mean of the initial 10 s images.
Calculate the fluorescence value for each image frame from the ROI.
Data analysis
Statistical analysis
Calcium transients were plotted as ∆F/F, where ∆F is the change in the fluorescence value from its baseline fluorescence (F).
The mean fluorescence intensities of the first 10 frames (t = 200 ms) were taken as baseline fluorescence (F0).
The odor stimulus was given for 20 s (40 frames). The analysis was conducted using GraphPad Prism 6 software.
Results
Immobilization of worms in alginate scaffold
2%, 3%, or 5% alginate polymer solutions were used to immobilize the worms. The viability of the worms was checked by counting the head bends per minute. No statistically significant difference in head bends was observed in worms trapped in 5% alginate compared to 2% and 3% alginate (p ≤ 0.5; n = 5; Figure 4A). However, when we converted the data as viability measurement, the results showed that there is a 100% probability of survival in 2% alginate compared to 80% and 60% in the 3% and 5% alginate scaffold, respectively (Figure 4B). A total immobilization of the worms was observed in the 5% alginate polymer compared to the other two percentages. To see the worm behavior in 2%, 3%, and 5% alginate polymer check supplementary Videos 1, 2, and 3, respectively.
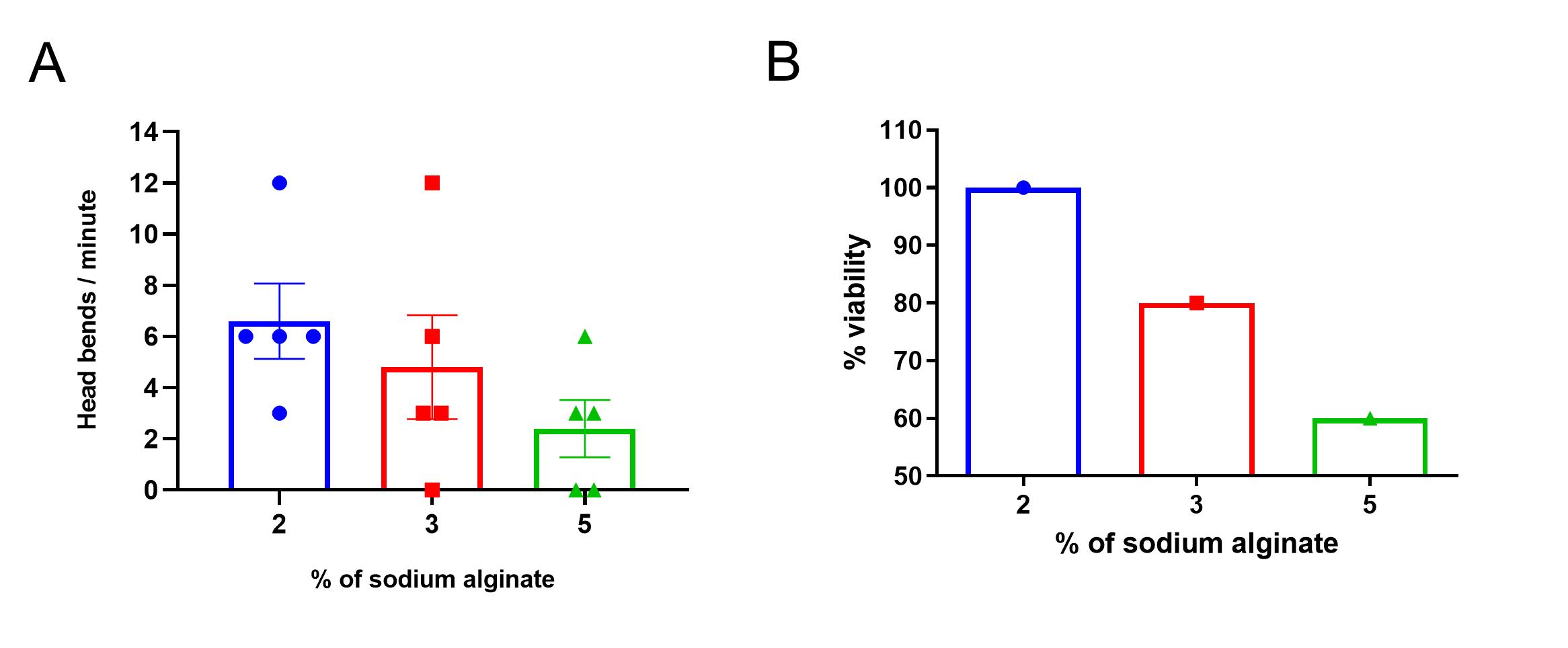
Figure 4. Viability of worms in sodium alginate gel. A. Head bends after immobilization. The head bends per minute for the 2%, 3%, and 5% alginate immobilized worms. Worms with no head bend within 1 min of observation were considered dead and not used in the study. B. Percentage of viability. The viability of the worms was 100% in 2% alginate, 80% in 3% alginate, and 60% in 5% alginate. The values are expressed as mean ± SEM; n = 5; ordinary one-way ANOVA.
Quantification of size alteration of worms in the alginate
The normal body width of C. elegans has a diameter of approximately 50 μm. We measured the worm's body width and concluded that the alginate polymer causes worm body expansion. Analysis was done in M9 buffer and different concentrations of alginate. The result showed that in the M9 buffer, the worm width is 60 μm. In comparison, in alginate immobilized worms, the width is approximately 80 μm (Figure 5).
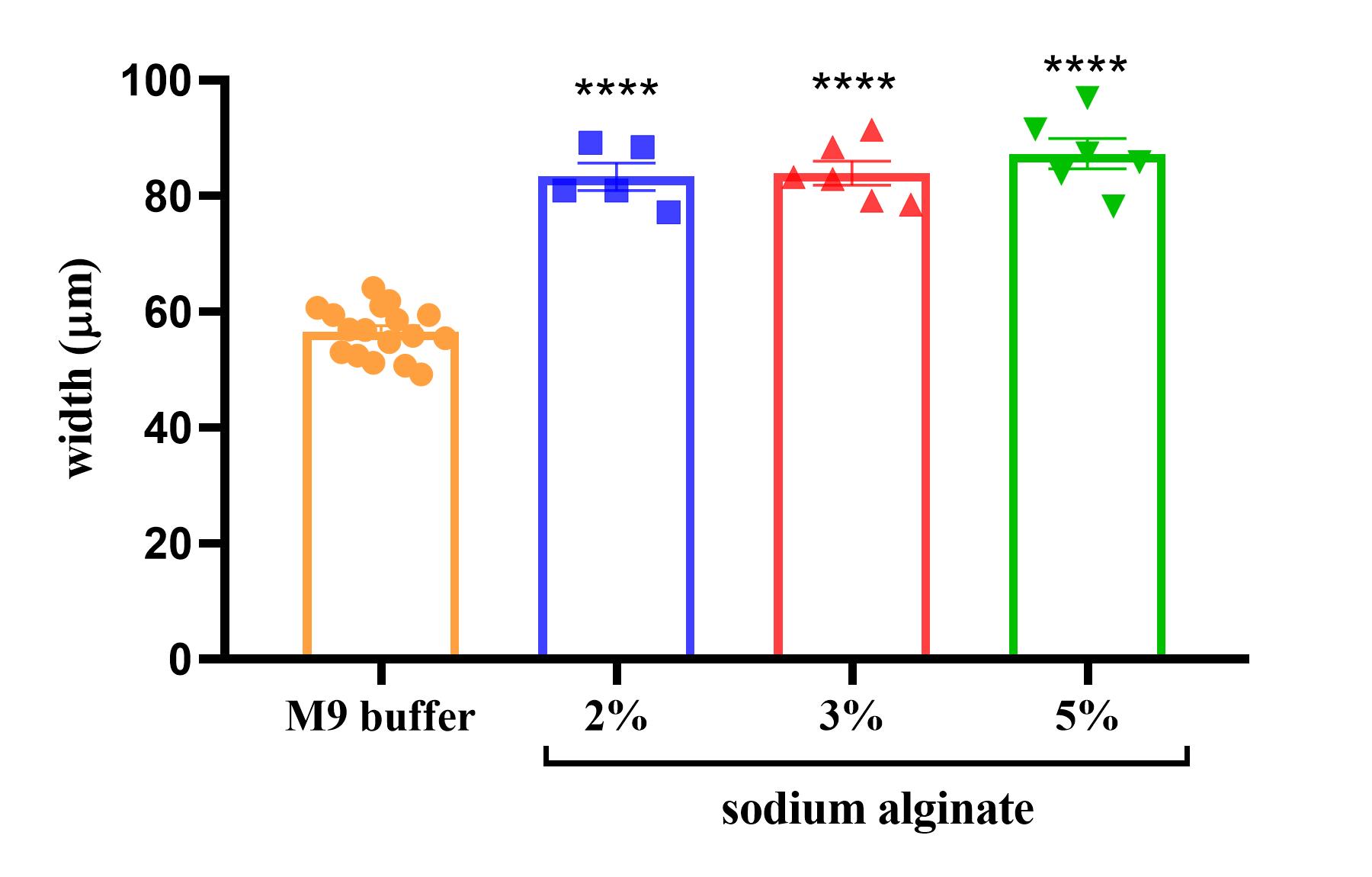
Figure 5. Body width of immobilized worms. Body width of the immobilized worms in different concentrations of sodium alginate. The values are expressed as mean ± SEM; n = 5 or more, ordinary one-way ANOVA, p < 0.0001 (****).
Measurement of RID neuron activation during the exposure of IAA
C. elegans shows attractive behavior towards IAA. Genetically encoded Ca2+ indicator GCaMP6-expressing (strain ZM9078 flp-14p::GCaMP6::wCherry) in RID was used to visualize Ca2+ transients in the RID interneuron, which helps for the forward movement while exposed to IAA. Calcium transients in the RID neuron were observed in the worm without and with exposure to 1/300 diluted IAA by entrapping the worm in the alginate polymer. The odor exposure lasted 20 s. Data showed a significant calcium influx in the RID interneuron during IAA exposure (Figure 6).
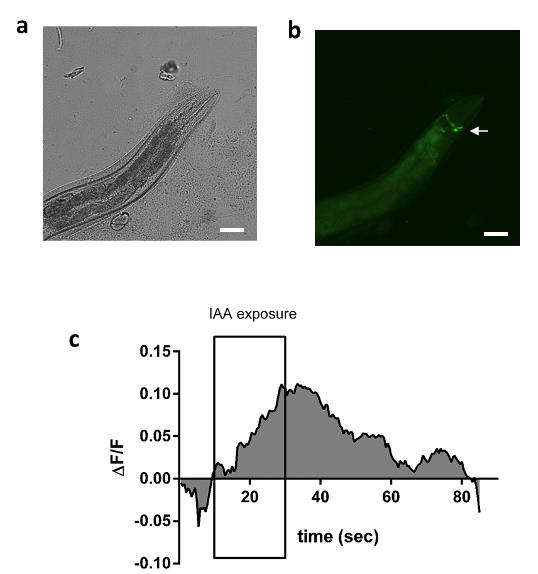
Figure 6. Calcium imaging of alginate immobilized worm. A. Brightfield image of the immobilized ZM9078 (hpIs587 - GCaMP6) strain. B. Fluorescent image of immobilized ZM9078 strain. C. Alginate-immobilized worms were used for calcium imaging. Worms were exposed to 1/300 diluted IAA for 20 s (data is an average of n = 7 readings).
Discussion and Conclusion
Compared with other imaging techniques in C. elegans, our in vivo imaging method is straightforward and affordable to image adult C. elegans without using any anesthetic agent or gluing the worm to the agar pad. Using this new method, we analyzed the neuronal calcium firing in worms by applying IAA as an odor stimulus. Significantly enhanced fluorescent intensity was observed in the ZM9078 strain during exposure to IAA. Time-lapse image analysis was done using Fiji software (Schindelin et al., 2012). Among the various percentages of sodium alginate tested, 5% alginate provided the best immobilization concentration.
The worms can be removed from the scaffold and kept back in the food plate for repeated imaging assays. The worms could survive for more than 30 min at room temperature in a 5% alginate scaffold or in a humid chamber for a couple of hours. Dehydration and starvation would significantly impact the survival of worms within the scaffold when they are kept for a prolonged period.
The change in body width was a significant observation while standardizing the protocol. The entrapment of the worms in the alginate scaffold could impact its size. The polymerization of alginate is a fast process and could reduce the space available for the worm, which could be one of the reasons for this observation.
Furthermore, we can deliver the volatile chemical stimuli from outside because of the high porosity of the alginate scaffold. Physiological and behavioral functions of the organism are not disturbed by alginate immobilization. The major limitation of this method is that entrapping in 5% alginate causes mortality in the worm. However, head bends can be used to select the surviving worms. One can try temperature control stages attached to the microscope to reduce the movements of the worms, which will allow using a lower alginate concentration to enhance the survival rate. With our approach, immobilizing C. elegans becomes much easier without any special technical knowledge.
Acknowledgments
We thank CGC for providing the ZM9078 strain. We acknowledge SCTIMST for supporting the research. We acknowledge Saurabh S. Nair, Department of Medical Device Engineering, SCTIMST for writing the Fiji macro files. We acknowledge SCTIMST for supporting the research. We also acknowledge CSIR for the fellowship to A.M. and UGC fellowship to R.S. The protocol is adapted from Raj and Thekkuveettil (2022).
Competing interests
The authors declare no conflict of interest.
References
- Dong, L., Cornaglia, M., Krishnamani, G., Zhang, J., Mouchiroud, L., Lehnert, T., Auwerx, J. and Gijs, M. A. M. (2018). Reversible and long-term immobilization in a hydrogel-microbead matrix for high-resolution imaging of Caenorhabditis elegans and other small organisms. PLoS One 13(3): e0193989.
- Fang-Yen, C., Gabel, C. V., Samuel, A. D., Bargmann, C. I. and Avery, L. (2012). Laser microsurgery in Caenorhabditis elegans. Methods Cell Biol 107: 177-206.
- Hulme, S. E., Shevkoplyas, S. S., Apfeld, J., Fontana, W. and Whitesides, G. M. (2007). A microfabricated array of clamps for immobilizing and imaging C. elegans. Lab Chip 7(11): 1515-1523.
- Kerr, R., Lev-Ram, V., Baird, G., Vincent, P., Tsien, R. Y. and Schafer, W. R. (2000). Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron 26(3): 583-594.
- Kim, E., Sun, L., Gabel, C. V. and Fang-Yen, C. (2013). Long-term imaging of Caenorhabditis elegans using nanoparticle-mediated immobilization. PLoS One 8(1): e53419.
- Lewis, J. A., Wu, C. H., Berg, H. and Levine, J. H. (1980). The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics 95(4): 905-928.
- Raj, V. and Thekkuveettil, A. (2022). Dopamine plays a critical role in the olfactory adaptive learning pathway in Caenorhabditis elegans. J Neurosci Res 100(11): 2028-2043.
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods. 28; 9(7):676-682.
Supplementary information
Video recording of worms entrapped in 2%, 3%, and 5% alginate polymer: Supplementary_videos_1-3.zip.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Mangalath, A., Raj, V., Santhosh, R. and Thekkuveettil, A. (2023). Alginate Gel Immobilization of Caenorhabditis elegans for Optical Calcium Imaging of Neurons. Bio-protocol 13(12): e4697. DOI: 10.21769/BioProtoc.4697.
Category
Neuroscience > Neuroanatomy and circuitry > Animal model
Neuroscience > Behavioral neuroscience
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








