- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Transformation and Detection of Soybean Hairy Roots
(*contributed equally to this work) Published: Vol 13, Iss 11, Jun 5, 2023 DOI: 10.21769/BioProtoc.4691 Views: 2574
Reviewed by: Zhibing LaiAli ParsaeimehrRaviraj Mahadeo Kalunke

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Simple and Fail-safe Method to Transform Miniprep Escherichia coli Strain K12 Plasmid DNA Into Viable Agrobacterium tumefaciens EHA105 Cells for Plant Genetic Transformation
Beenzu Siamalube [...] Steven Runo
Jan 5, 2025 2340 Views
A Novel Gene Stacking Method in Plant Transformation Utilizing Split Selectable Markers
Guoliang Yuan [...] Xiaohan Yang
Feb 20, 2025 1967 Views
Highly Efficient Agrobacterium-Mediated Transformation of Tomato cv Micro-Tom From Cotyledon Explants
Débora Pagliuso [...] Luciano Freschi
Dec 5, 2025 1449 Views
Abstract
Agrobacterium rhizogenes is a soil bacteria with extensive infectivity, which can infect almost all dicotyledonous plants and a few monocotyledonous plants to induce root nodules. This is caused by the root-inducing plasmid, which contains genes responsible for the autonomous growth of root nodules and crown gall base synthesis. Structurally, it is similar to the tumor-inducing plasmid in that it mainly contains the Vir region, the T-DNA region, and the functional region of crown gall base synthesis. Its T-DNA is integrated into the nuclear genome of the plant with the assistance of Vir genes, causing hairy root disease in the host plant and the formation of hairy roots. The roots produced by Agrobacterium rhizogenes–infested plants are characterized by a fast growth rate, high degree of differentiation, physiological, biochemical, and genetic stability, and ease of manipulation and control. In particular, the hairy root system is an efficient and rapid research tool for plants that have no affinity for transformation by Agrobacterium rhizogenes and low transformation efficiency. The establishment of germinating root culture system for the production of secondary metabolites in the original plants through the genetic transformation of natural plants mediated by root-inducing plasmid in Agrobacterium rhizogenes has become a new technology combining plant genetic engineering and cell engineering. It has been widely used in a variety of plants for different molecular purposes, such as pathological analysis, gene function verification, and secondary metabolite research. Chimeric plants obtained by induction of Agrobacterium rhizogenes that can be expressed instantaneously and contemporarily are more rapidly obtained, compared to tissue culture and stably inheritable transgenic strains. In general, transgenic plants can be obtained in approximately one month.
Keywords: Agrobacterium rhizogenesBackground
The methods for soybean genetic transformation can be roughly divided into two types, with different applications: (1) Agrobacterium-mediated transformation, and (2) foreign target genes being introduced into the recipient soybean cells by auxiliary means, such as instruments (gene guns) or reagents (cell wall enzymes). This second type includes the microinjection, pollen tube channel, electroporation (Zhou and Qiu, 2010), polyethylene glycol (Liu and Friesen, 2012), and gene gun methods.
Agrobacterium rhizogenes–mediated genetic transformation is a new plant tissue culture technology that emerged in the late 20th century. As A. rhizogenes can induce hairy roots without hormone promotion and resistance screening, it has been widely used in the research of many plants for multiple molecular purposes, such as pathological analysis, gene function verification, secondary metabolite research, etc. (Fathi et al., 2019; Jiang et al., 2019; Meng et al., 2019). Reported plants that can be induced into hairy roots by A. rhizogenes include soybean, mesembryanthemum crystalline, white campion, and Salix (Gomes et al., 2019; Hudzieczek et al., 2019; Hwang et al., 2019; Yu et al., 2021; Zhang et al., 2022; Zhao et al., 2022).
In this protocol, we improved the method of genetic transformation of soybean mediated by A. rhizogenes, to establish various genetic transformation systems for inducing hairy roots in soybean and to provide technical support for soybean molecular biology research.
Materials and reagents
Soybean seeds (William 82) (Glycine max) or other soybean varieties suitable for transformation
Agrobacterium rhizogenes K599 chemically competent cell (Beijing Zoman Biotechnology Co., Ltd. catalog number: ZC1506)
pCAMBIA3301 or other plasmid (CAMBIA)
NcoI and BstEII (Thermo Fisher Scientific, Waltham, MA, USA)
Glufosinate-ammonium (Basta®) (Shanghai Acmec Biochemical Co., Ltd, catalog number: A607805g)
Kanamycin solution (100 mg/L) (Inalco Pharmaceuticals. catalog number: 1758-9316)
Streptomycin solution (100 mg/L) (Inalco Pharmaceuticals. catalog number: 1758-9319)
GUS stain solution (Biorigin Inc, catalog number: BN20175)
Tryptone (OXOID LIMITED, catalog number: LP0042B)
Yeast extract (OXOID LIMITED, catalog number: LP0021T)
NaCl (Beijing Biomed Gene Technology Co., Ltd. catalog number: SH5001-01)
CaCl2·2H2O (Sigma-Aldrich catalog number: C7902-500G)
90% acetone (Sigma-Aldrich catalog number: 270725-500mL)
Glycerol
Liquid and solid LB medium (for 1 L) (see Recipes)
0.1 M CaCl2 solution (see Recipes)
Equipment
Disposable plastic Petri dishes (Beijing Ruiaizhengte Biotechnology Co., Ltd)
Medical disposable sterile syringe
Gauze
Disposable transparent cup
1 L culture flasks
Procedure
Preparation of soybean seeds
Select full and uniform seeds, removing any that are diseased, small, or damaged. Prepare a suitable number of clean Petri dishes with water-moistened gauze, place the selected seeds in the clean Petri dishes, spread the moistened gauze on top of the soybean seeds, cover the Petri dishes, and incubate in the dark for 12 h.
Before transferring the seeds to the nutrient soil, pre-moisten the soil with water, fill each pot with an equal amount of pre-moistened wet soil, and press the pots gently to flatten the soil. Next, place the absorbed seeds evenly on top of the pressed soil. Then, lightly cover each pot with a layer of remaining soil as needed. Spray each pot with an appropriate volume of water using a spray bottle.
Incubate in a greenhouse at 28 °C for 5–7 days until 10 cm seedlings have grown for the next transformation step.
Generation of Agrobacterium rhizogenes competent cells
Take frozen K599 chemically competent cells stored at -80 °C and dip them gently into a sterile inoculation loop on a UV-irradiated ultra-clean table. Draw a line on an antibacterial-free plate and then incubate for 16–20 h at 37 °C.
Take a single colony and inoculate it in 5 mL of LB liquid medium. Oscillate at 220 rpm at 28 °C for 12–16 h.
Transfer 2 mL of bacterial solution into 100 mL of LB liquid medium and incubate at 28 °C at 220 rpm until OD600 = 0.5.
Transfer to a sterile centrifuge tube and centrifuge at 3,000× g for 5 min. Remove the supernatant.
Add 10 mL of pre-cooled 0.1 M CaCl2 solution, gently suspend the cells, and place on ice for 20 min. Centrifuge at 3,000× g for 5 min at 4 °C and remove the supernatant.
Add 4 mL of pre-chilled 0.1 M CaCl2 solution containing 15% glycerol and gently suspend.
Dispense Agrobacterium rhizogenes competent cells in sterile Eppendorf tubes, each tube with 200 μL. Freeze at -80 °C.
Preparation of A. rhizogenes
Insert target gene into the pCAMBIA3301 vector with kanamycin resistance with a phosphinothricin selection marker from both NcoI and BstEII enzymatic cut sites. In this vector, the lac promoter drives the expression of the inserted gene. To screen transformed roots, the vector contains a promoter encoding an enhanced glufosinate resistance gene derived from the cauliflower mosaic virus gene CaMV control, which provides strong root expression. The gene encoding kanamycin resistance provides antibiotic selection. Transfer the fusion expression vector plasmid with the target gene into A. rhizogenes K599 by the freeze-thaw method.
Place the K599 competent cells stored at -80 °C at room temperature or in an ice water bath for a moment and wait for its partial melting. Just-thawed cells have the highest conversion efficiency.
Add 1 μg of plasmid DNA per 100 μL of competent cells and mix well by gently toggling the bottom of the tube with your hands. Then, let it stand on ice for 5 min, liquid nitrogen for 5 min, 37 °C water bath for 5 min, and ice bath for 5 min, in this order.
Add 800 μL of LB liquid medium without antibiotics and incubate at 28 °C at 200 rpm for 2–3 h.
Centrifuge at 3,000× g for 1 min at room temperature. Leave 100 µL of the supernatant to resuspend the remaining bacteria onto LB plates containing the appropriate antibiotics and incubate upside down in an incubator at 28 °C for 2–3 days.
Induction of hairy roots
Prepare sterile disposable syringes. Puncture (5 mm) the cotyledons of young soybean seedlings with a syringe to infect the (approximately 7-days-old) soybean seedlings with A. rhizogenes containing plant expression vectors (Figure 1). Prepare a sterile disposable syringe, scrape the colonies on the plate transformed with the target gene and collect them with a needle tip. Then, pass the needle through the cotyledon hypocotyl (Figures 2 and 3) to stab the plant, ensuring that the needle passes through the central part of the hypocotyl. Place a clear plastic cup (with approximately 20 cm in height) upside down above the seedlings to facilitate moisture retention and thus improve the efficiency of infection, while incubating for 24 h under light-proof conditions.
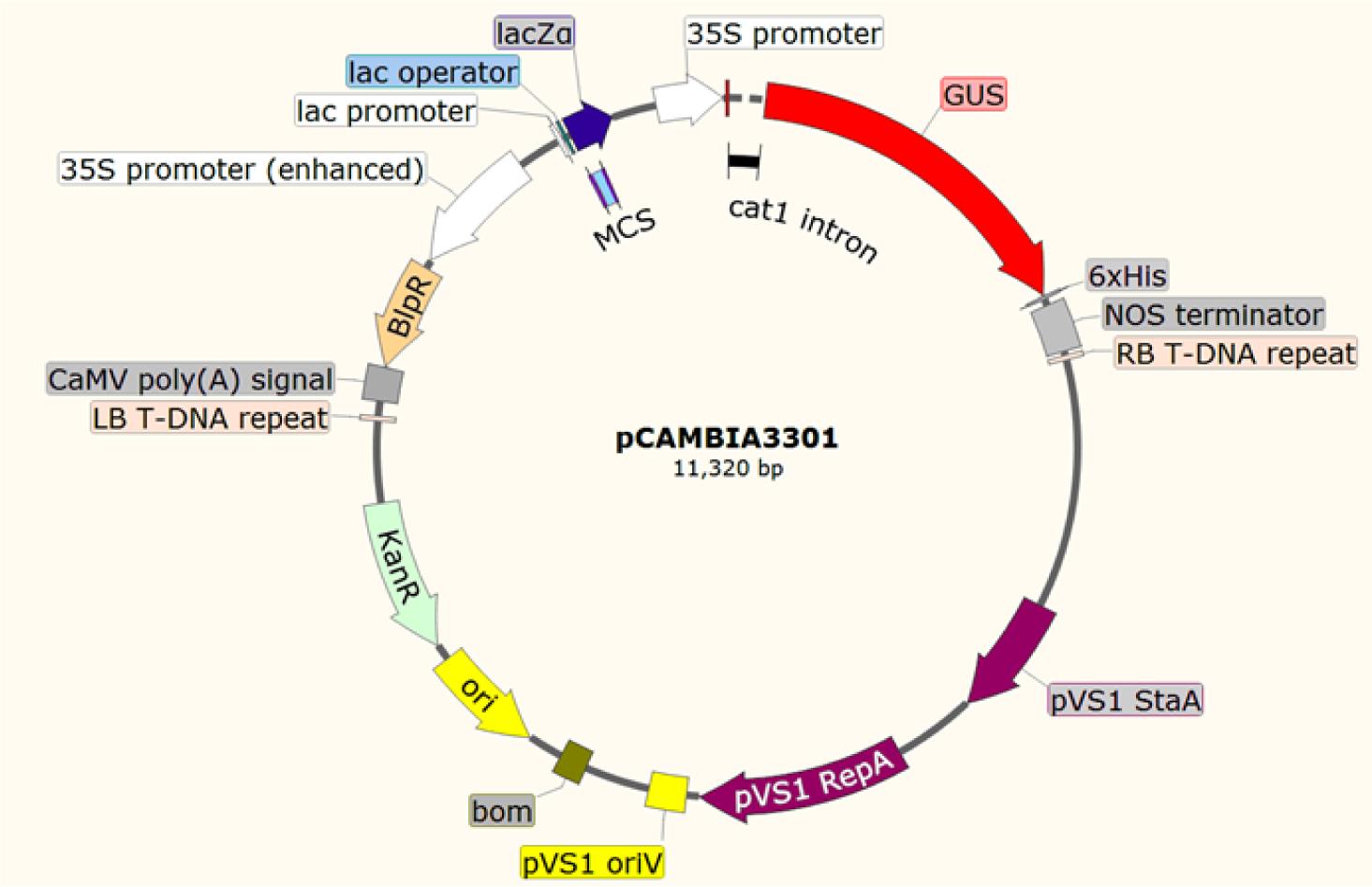
Figure 1. Vector contains kanamycin resistance gene for bacterial selection and bialaphos resistance (BlpR) gene for plant selection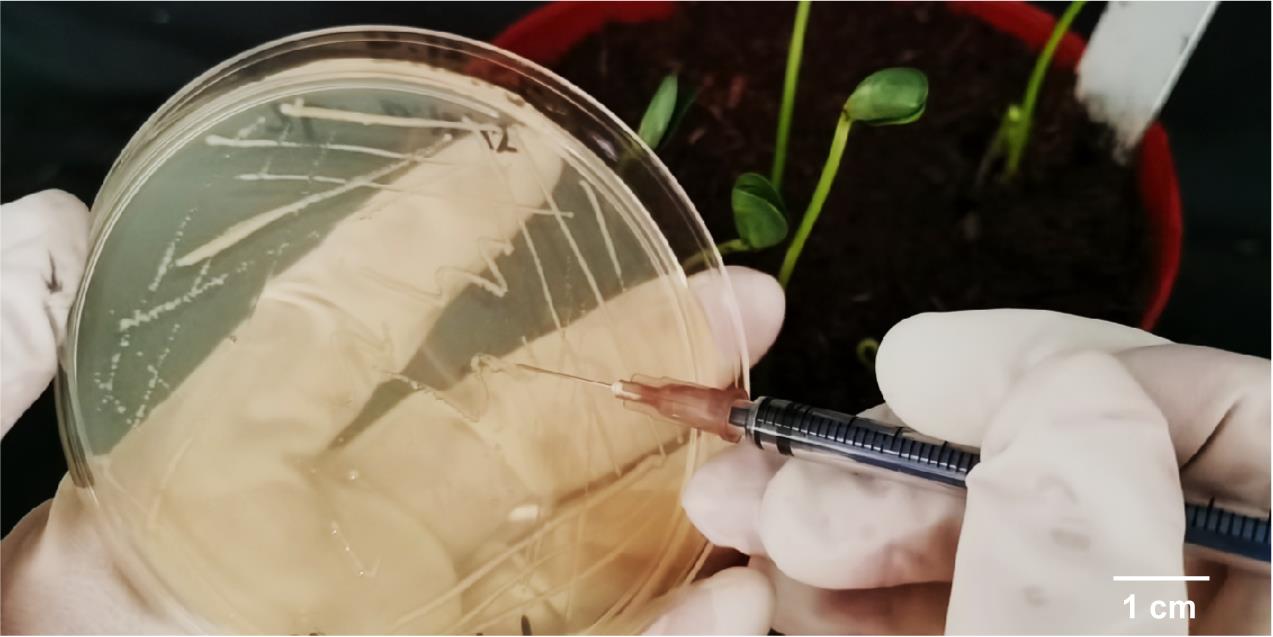
Figure 2. Inoculation with bacterial paste
Figure 3. Stabbing of the hypocotyl close to the cotyledonary nodeThen, grow under normal conditions (25 °C; 16:8 h light/dark cycle). Cut off the bottom of the cup, put it upside down on the plant to fix the soybean plant, cover the cup with soil to completely cover the infected part, and pour sufficient water.
After approximately two weeks, observe whether new roots have grown at the infected location (Figure 4). During this period, apply sufficient water and fertilizer to ensure normal plant growth and promptly remove any damaged and dead plants.

Figure 4. New roots growing at the infected locationAt the two-leaf stage, directly spray glufosinate-ammonium to the plants to be selected. (Different plants have different sensitivities to Basta®, and the same plant has different sensitivities at different growth stages. Criteria for determining the critical screening concentration: Basta® concentration at which wildtype plants can hardly germinate, or germinate but then cannot survive. The optimal screening concentration of Basta® is determined according to the yellowing degree and mortality rate of soybean seeds.)
Note: The general working concentration of spraying selected plants is 0.001%–0.002%.
Alternatively, the expression of the reporter gene can be detected by GUS staining. The specific procedures are as follows:
Pretreatment: cut the roots into small pieces, put them in a 1.5 mL centrifuge tube, add pre-cooled 90% acetone to completely cover the material, and treat at room temperature for 20–30 min. This step can pre-fix the tissue and remove some chlorophyll.
Staining: rinse the material with distilled water and place in a 1.5 mL centrifuge tube; add an appropriate volume of prepared GUS stain solution until the material is completely covered, wrap in aluminum foil, and leave overnight at room temperature.
Elution: Gradient elution with 25%, 50%, 70%, and 95% ethanol with gentle shaking on a shaker for 20 min each time.
Observation: The blue dots on the white background are the GUS expression sites when observed with the naked eye or under a microscope.
Seven days later, when the hairy root is approximately 5–10 cm (long enough to support the normal growth of the plant), select plants with consistent growth and cut the hypocotyl approximately 1 cm below the wound formed by the hairy root. Remove the primary root, retain the new root, and transplant it to a new basin.
Recipes
Liquid and solid LB medium (for 1 L)
Tryptone 10 g
Yeast extract 5 g
NaCl 10 g
Dissolve in 1 L of double-distilled water; then, sterilize by autoclaving at 120 °C for 20 min
0.1 M CaCl2 solution
Weigh 0.28 g of CaCl2·2H2O, dissolve in 50 mL of double-distilled water, fix the volume to 100 mL, and autoclave.
Acknowledgments
This protocol is adapted from our previous work (Zhang et al., 2022). This research was financially supported by the National Key R&D Program of China (2022YFD1201700), Hainan Yazhou Bay Seed Lab (B21HJ0215) and Nanfan special project, CAAS (YBXM04).
Competing interests
There are no conflicts of interest or competing interests.
References
- Fathi, R., Mohebodini, M. and Chamani, E. (2019). High-efficiency Agrobacterium rhizogenes-mediated genetic transformation in Cichorium intybus L. via removing macronutrients. Ind Crops Prod 128: 572-580.
- Gomes, C., Dupas, A., Pagano, A., Grima-Pettenati, J. and Paiva, J. A. P. (2019). Hairy Root Transformation: A Useful Tool to Explore Gene Function and Expression in Salix spp. Recalcitrant to Transformation. Front Plant Sci 10: 1427.
- Hudzieczek, V., Cegan, R., Cermak, T., Bacovska, N., Machalkova, Z., Dolezal, K., Plihalova, L., Voytas, D., Hobza, R. and Vyskot, B. (2019). Agrobacterium rhizogenes-mediated transformation of a dioecious plant model Silene latifolia. N Biotechnol 48: 20-28.
- Hwang, H. H., Wang, C. H., Chen, H. H., Ho, J. F., Chi, S. F., Huang, F. C. and Yen, H. E. (2019). Effective Agrobacterium-mediated transformation protocols for callus and roots of halophyte ice plant (Mesembryanthemum crystallinum). Bot Stud 60(1): 1.
- Jiang, H., Li, K. and Gai, J. (2019). Agrobacterium rhizogenes-induced soybean hairy roots versus Soybean mosaic virus (ARISHR-SMV) is an efficient pathosystem for studying soybean-virus interactions. Plant Methods 15: 56.
- Liu, Z. and Friesen, T. L. (2012). Polyethylene glycol (PEG)-mediated transformation in filamentous fungal pathogens. Methods Mol Biol 835: 365-375.
- Meng, D., Yang, Q., Dong, B., Song, Z., Niu, L., Wang, L., Cao, H., Li, H. and Fu, Y. (2019). Development of an efficient root transgenic system for pigeon pea and its application to other important economically plants. Plant Biotechnol J 17(9): 1804-1813.
- Yu, T. F., Liu, Y., Fu, J. D., Ma, J., Fang, Z. W., Chen, J., Zheng, L., Lu, Z. W., Zhou, Y. B., Chen, M., Xu, Z. S. and Ma, Y. Z. (2021). The NF-Y-PYR module integrates the abscisic acid signal pathway to regulate plant stress tolerance. Plant Biotechnol J 19(12): 2589-2605.
- Zhang, H. Y., Hou, Z. H., Zhang, Y., Li, Z. Y., Chen, J., Zhou, Y. B., Chen, M., Fu, J. D., Ma, Y. Z., Zhang, H. and Xu, Z. S. (2022). A soybean EF-Tu family protein GmEF8, an interactor of GmCBL1, enhances drought and heat tolerance in transgenic Arabidopsis and soybean. Int J Biol Macromol 205: 462-472.
- Zhao, J., Zheng, L., Wei, J., Wang, Y., Chen, J., Zhou, Y., Chen, M., Wang, F., Ma, Y. and Xu, Z. S. (2022). The soybean PLATZ transcription factor GmPLATZ17 suppresses drought tolerance by interfering with stress-associated gene regulation of GmDREB5. Crop J 10(4): 1014-1025.
- Zhou, G. A. and Qiu, L. J. (2010). Identification and Functional Analysis on Abiotic Stress Response of Soybean Cl− Channel Gene GmCLCnt. Agricultural Sciences in China 9(2): 199-206.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Xu, X., Yu, T. F., Ma, J., Chen, J., Zhou, Y. B., Chen, M., Chen, Z. Y., Ma, Y. Z., Xu, Z. S. and Zhang, Z. A. (2023). Transformation and Detection of Soybean Hairy Roots. Bio-protocol 13(11): e4691. DOI: 10.21769/BioProtoc.4691.
Category
Plant Science > Plant transformation > Agrobacterium
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








