- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Modified Pseudo-Schiff Propidium Iodide for Staining the Shoot Apical Meristem in Arabidopsis
Published: Vol 13, Iss 9, May 5, 2023 DOI: 10.21769/BioProtoc.4672 Views: 2279
Reviewed by: Ansul LokdarshiRicardo Urquidi CamachoPablo Bolanos-Villegas

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantitative Live Confocal Imaging in Aquilegia Floral Meristems
Ya Min [...] Elena M. Kramer
Jun 20, 2022 3608 Views

A Novel Imaging Protocol for Investigating Arabidopsis thaliana Siliques and Seeds Using X-rays
Brylie A. Ritchie [...] Ansul Lokdarshi
Oct 5, 2023 2195 Views

Direct Plant Regeneration From Immature Male Inflorescence of Banana (Musa spp.)
Pradeep Chand Deo
Oct 20, 2025 1450 Views
Abstract
Visualization of cell structure with fluorescent dye for characterizing cell size, shape, and arrangement is a common method to study tissue morphology and morphogenesis. In order to observe shoot apical meristem (SAM) in Arabidopsis thaliana by laser scanning confocal microscopy, we modified the pseudo-Schiff propidium iodide staining method by adding a series solution treatment to stain the deep cells. The advantage of this method is mainly reflected by the direct observation of the clearly bounded cell arrangement and the typical three-layer cells in SAM without the traditional tissue slicing.
Keywords: Shoot apical meristem (SAM)Background
Most of the above-ground plant organs are derived from the shoot apical meristem (SAM) (Steeves and Sussex, 1989; Dinneny and Benfey, 2008). As a key tissue involved in plant growth and development, it plays a role in maintaining the ability of stem cells to divide continuously (Fletcher, 2018; Hu et al., 2018), controlling the initiation of organs development (Spinelli et al., 2011; Balkunde et al., 2017), accurately regulating the spatio-temporal specific gene expression (Mayer et al., 1998; Long et al., 1996; Williams, 2021), and integrating the signals from various cell types (Zhao et al., 2010; Azizi et al., 2015; Chung et al., 2019). Thus, exploring the mechanism of complex regulation patterns in the meristem is inseparable from observation of meristem at the cellular level.
Unlike the inflorescence meristem, the SAM of Arabidopsis is tightly wrapped by the surrounding leaf primordia and leaves, which makes it difficult to observe its cellular structure in its deeper part (Moreno et al., 2006). Scientists usually observe the cell structure and morphogenesis of SAM by semithin sections or tissue clearing, but only one cell layer or the surface layer of the SAM is observed (Koi and Kato, 2007; Zhang et al., 2021; Wang et al., 2022). To visualize three-dimensional structure of plant tissues, scientists prefer to use fluorescent dyes, such as propidium iodide, to stain tissues for optical section for laser scanning confocal microscopy (Haseloff, 2003; Truernit et al., 2008; Prunet et al., 2016; Shi et al., 2016).
Previously, we observed the cell structure of SAM and inflorescence meristem in Arabidopsis by modifying the pseudo-Schiff propidium iodide staining method (Li et al., 2022). Compared with traditional methods, this method presents several advantages. First, it allows the observation of the different cell layers in the whole SAM, which is a continuous and complete structure. Second, the cellular structure of the initial leaf primordium is observable except for the SAM. Third, unlike the traditional method that takes seven days, this method is simple in terms of procedure and time saving, taking only three days to complete. It can be foreseen that this method will likely be applicable to the observation of other tissues or SAM of other plant species. The operation details are described in the following section.
Materials and Reagents
1.5 mL centrifuge tubes (Chenglin, catalog number: CL22091809)
Microscope slides (CITOTEST, catalog number: 10127101P-G)
Microscope cover glass (CITOTEST, catalog number: 10212222C)
Qualitative filter paper (Beimu, catalog number: 101)
60 mm round culture dishes (Jindian, catalog number: 16021-1)
Aluminum foil for experiment (Zaowufang, catalog number: 615)
1 mL syringe (Zhi Yu, catalog number: V500111)
Single edge blades (Personna, catalog number: 84-0701)
Sodium hypochlorite solution (Aladdin, catalog number: S101636)
Murashige and Skoog basal medium (Sigma, catalog number: M5519)
Sucrose (Hushi, catalog number: 10021418)
Potassium hydroxide (Hushi, catalog number: 10017008)
Methanol (Aladdin, catalog number: M116115)
Acetate (Aladdin, catalog number: A116172)
Ethanol (Aladdin, catalog number: E130059)
Hydrochloric acid (Aladdin, catalog number: H399657)
Sodium hydrogen bisulfite (Hushi, catalog number: 10019018)
Phytagel (Sigma, catalog number: P8169)
Periodic acid (Sigma, catalog number: P0430)
Propidium iodide (Sigma-Aldrich, catalog number: P4170)
Chloral hydrate C-IVN (Sigma-Aldrich, catalog number: 15307)
Glycerol (Sigma-Aldrich, catalog number: G7893)
Gum arabic from acacia tree (Sigma, catalog number: G9752)
Solid MS medium (see Recipes)
Fixative solution (see Recipes)
1% periodic acid solution (see Recipes)
Pseudo-Schiff propidium iodide solution (see Recipes)
Chloral hydrate solution (see Recipes)
Hoyer′s solution (see Recipes)
Ethanol solutions (see Recipes)
Equipment
Tweezers (Venus, #5)
Dissecting microscope (Zeiss, model: Axio Zoom V16)
Laser scanning confocal microscope (Zeiss, model: 780)
Growth chamber (Percival, model: CU36L5)
Fume hood
Clean bench
Procedure
Arabidopsis seedlings growth
Soak the Arabidopsis seeds with 1 mL of ddH2O in a 1.5 mL centrifuge tube and place the tube at room temperature for 1 h.
Pour out all the liquid and sterilize the Arabidopsis seeds with 15% sodium hypochlorite solution. Shake the tube by hand and let it soak thoroughly for 10 min.
On a sterile clean bench, pour out the sodium hypochlorite solution from the tube and wash the seeds 3–5 times with 1 mL of sterile ddH2O. Then, place the seeds at 4 °C for three days to break dormancy.
On a sterile clean bench, place the seeds onto round culture dishes with solid MS medium (see Recipes), and seal plates with micropore tape. Preparing 50 seedlings per condition is recommended for subsequent experiments.
Place the solid MS medium plate in the growth chamber under 16 h/22 °C light and 8 h/18 °C darkness for nine days (or any desired growth conditions). Use 9-day-old seedlings for subsequent experiments.
SAM dissection
Using a single edge blade, cut the cotyledons and root to separate the other tissue from the shoot and leave the hypocotyl connected to the shoot tips (Figure 1A and 1B).
Add 30 shoot tips to the centrifuge tube containing freshly prepared fixative solution (see Recipes) and leave at 4 °C overnight (Figure 1C).
Pour out the fixative solution and wash the shoot tips with ddH2O twice.
Pour out the water and add 30% ethanol solution for 15 min. Repeat this step with 50%, 70%, 80%, 90%, 95%, and 100% ethanol series (see Recipes).
Note: At this dehydrated stage, the shoot tips will turn white from the ethanol treatment (Figure 1D).

Figure 1. Preparation of shoot tips. A. Dissecting microscope images of the 9-day-old plant. Scale bar: 1,000 μm. B. Dissecting microscope images of the shoot tips with hypocotyl. Scale bar: 1,000 μm. White dashed lines mark the base of petiole. Cut the cotyledons and root along the dashed lines using a single edge blade. C. Centrifuge tube containing shoot tips (green) in fixative solution. D. Centrifuge tube containing shoot tips (white) in 100% ethanol.Transfer the shoot tips to new 100% ethanol overnight at room temperature.
Note: At this stage, you can keep the shoot tips in ethanol at -20 °C for several weeks.
Transfer the shoot tips to the 60 mm round culture dishes with 100% ethanol and place the culture dishes under the dissecting microscope (Figure 2A). Then, grip the hypocotyl with tweezers (Figure 2B) and remove the tiny true leaves from the shoot tip using a syringe needle under the dissecting microscope (Figure 2C–2E). Subsequently, transfer the shoot tips to the 1.5 mL centrifuge tube with 100% ethanol.
Note: This sample is very small and easily broken; be sure to gently wash the sample with solution in the subsequent experiment.
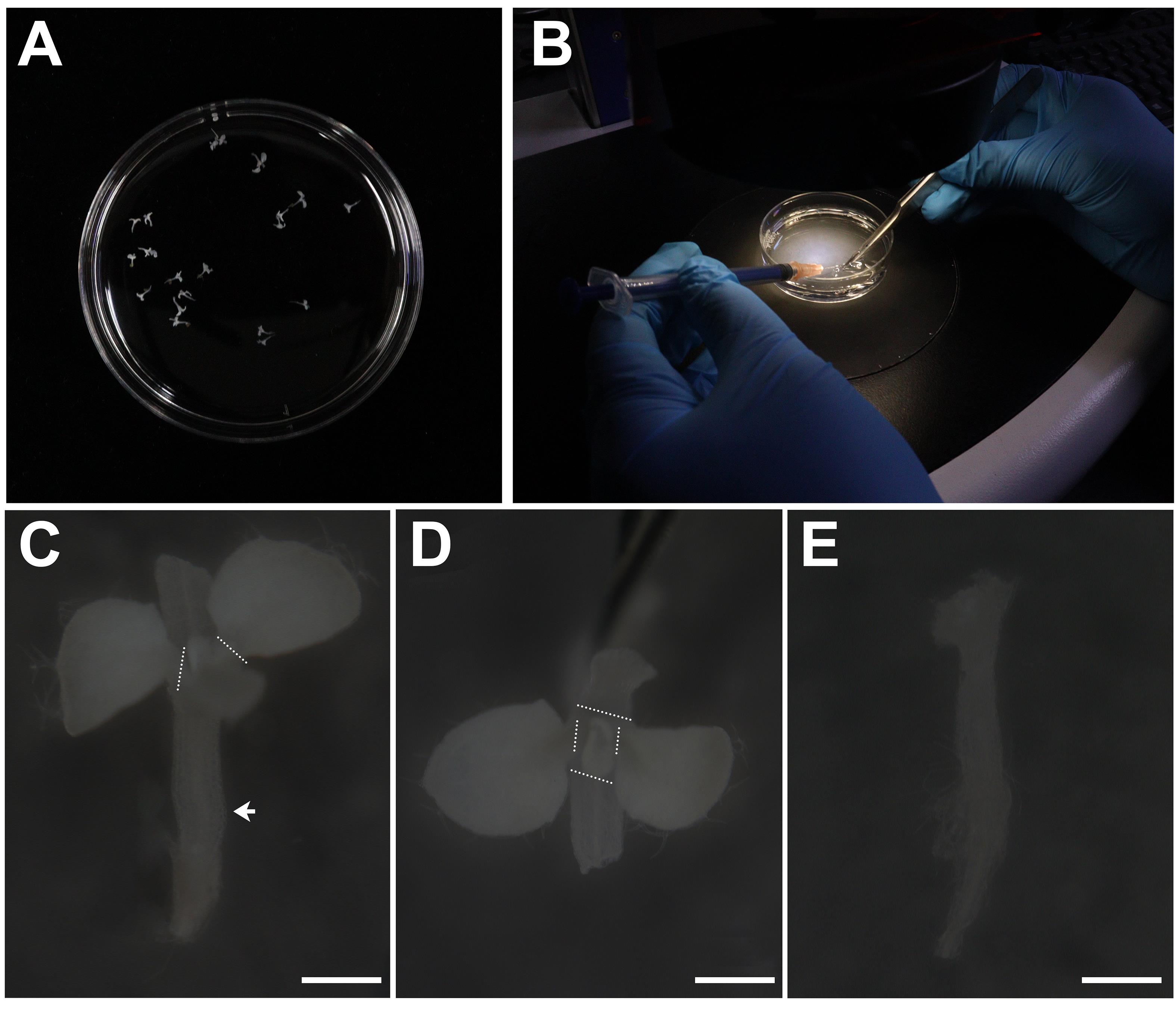
Figure 2. Dissection of shoot tips. A. Round culture dish containing shoot tips in 100% ethanol. B. Dissection of shoot tips using a syringe needle or tweezers under the dissecting microscope. C. Dissecting microscope images of the shoot tips before dissection. The arrow indicates the hypocotyl; white dashed lines mark the base of petiole. Scale bar: 500 μm. D. Images of the shoot tips from the top before dissection. White dashed lines mark the base of petiole. Scale bar: 500 μm. Grip the hypocotyl of shoot tips with tweezers and remove the leaves along the dashed line using a syringe needle or tweezers. E. Dissecting microscope images of the shoot tips after dissection. Scale bar: 500 μm.
Staining of SAM
Remove the 100% ethanol carefully and add 95% ethanol solution for 15 min. Repeat this step with 90%, 80%, 70%, 50%, 30%, and 15% ethanol series and ddH2O. Then, wash the shoot tips with ddH2O once.
Remove the water carefully and add 1 mL of 1% periodic acid solution (see Recipes) for 40 min. Then, wash the shoot tips with ddH2O twice.
Remove the water carefully and add 1 mL of pseudo-Schiff propidium iodide solution (see Recipes) for 2 h. Wrap the centrifuge tube with aluminum foil to protect the shoot tips from light.
Note: At this stage, the shoot tips will turn pink from the pseudo-Schiff propidium iodide solution. Protect the shoot tips from direct light in subsequent experiments (Figure 3A).
Carefully remove the pseudo-Schiff propidium iodide solution and wash the shoot tips with ddH2O twice.
Note: Because propidium iodide is toxic, discarded pseudo-Schiff propidium iodide solution requires a special waste container.
Transfer the shoot tips to the microscope slide and blot dry the liquid around the sample with qualitative filter paper. Then, drop 10–20 μL of chloral hydrate solution (see Recipes) and leave for 20–30 min at room temperature, away from light (Figure 3C).
Note: At this stage, the shoot tips will become transparent from chloral hydrate solution treatment (Figure 3B).
Remove the chloral hydrate solution with qualitative filter paper and drop 100 μL of Hoyer′s solution (see Recipes) to cover the shoot tips. Then, carefully lay down a microscope cover glass, avoiding air bubbles (Figure 3D).
Place the slides for three days at room temperature away from light. You can keep the slides at room temperature for several weeks.
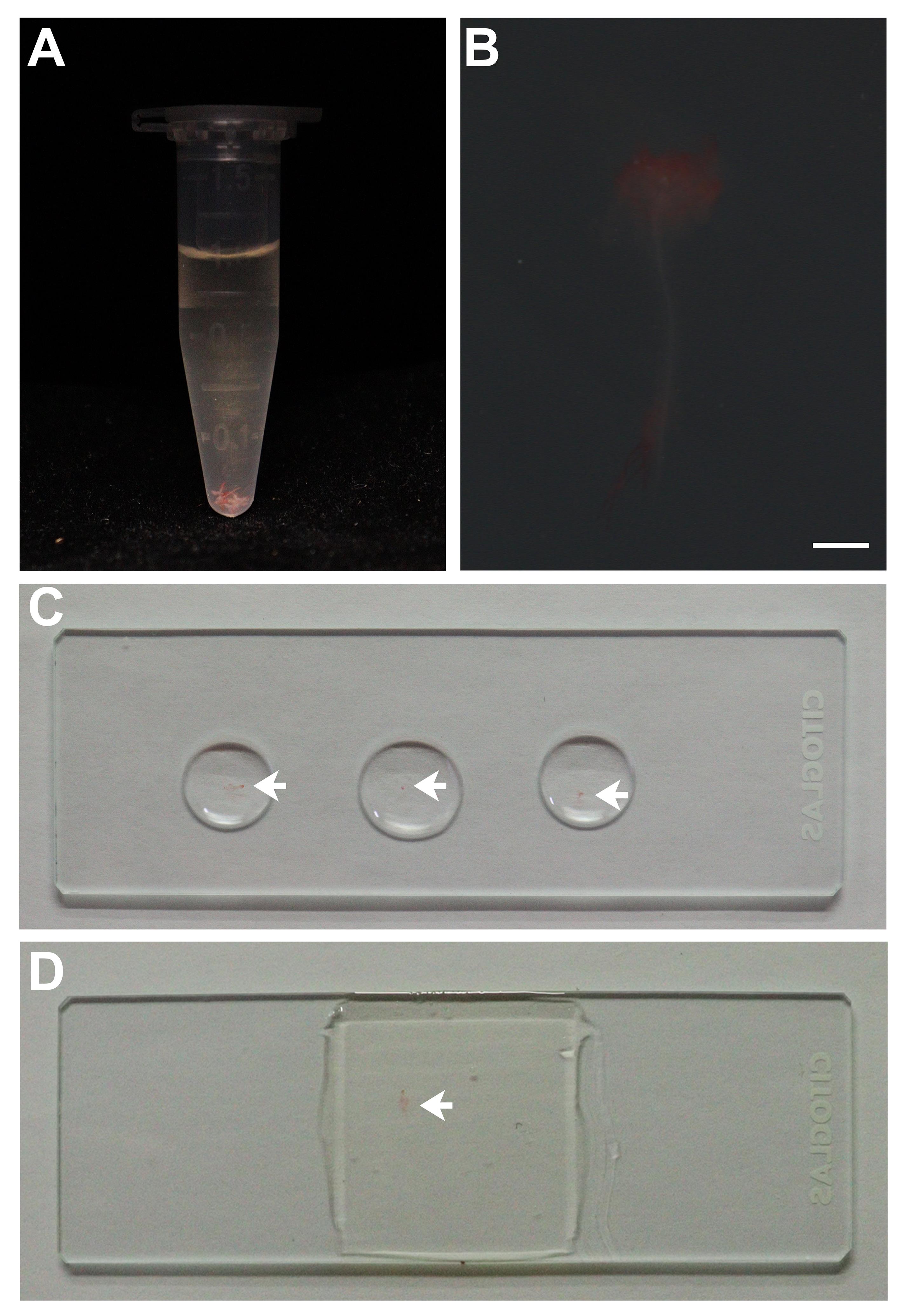
Figure 3. Staining of shoot tips. A. Centrifuge tube containing shoot tips in pseudo-Schiff propidium iodide solution. The shoot tips are pink. B. Dissecting microscope images of the shoot tips during chloral hydrate solution treatment. Scale bar: 500 μm. C. Microscope slide with shoot tips in chloral hydrate solution. The arrow indicates the shoot tip. D. Microscope slide with shoot tips in Hoyer′s solution. The arrow indicates the shoot tip.
Imaging of SAM
Observe the shoot tips under a Zeiss 780 laser scanning confocal microscope with a microscope objective (Plan-apochromat 40×/1.3 Oil DIC M27).
Set channel parameters as: excitation wavelength 514 nm; emission wavelength 566–658 nm; intensity of laser approximately 20%.
Set the zoom 5× to magnify the image; resolution is 1,024 × 1,024. Set speed of scanning parameter as 7. Observe and image the different cell layers in the whole SAM by adjusting the z-axis. If you want a layer of cells, set the scan mode to x-y recording; if you want images of a complete SAM, set the scan mode to z-stack collection. In the x, y, z collection mode, set the parameter “line averaging” to achieve nice quality. In the central layer of SAM (Z10), you can observe typical three-layer cells (Figure 4).
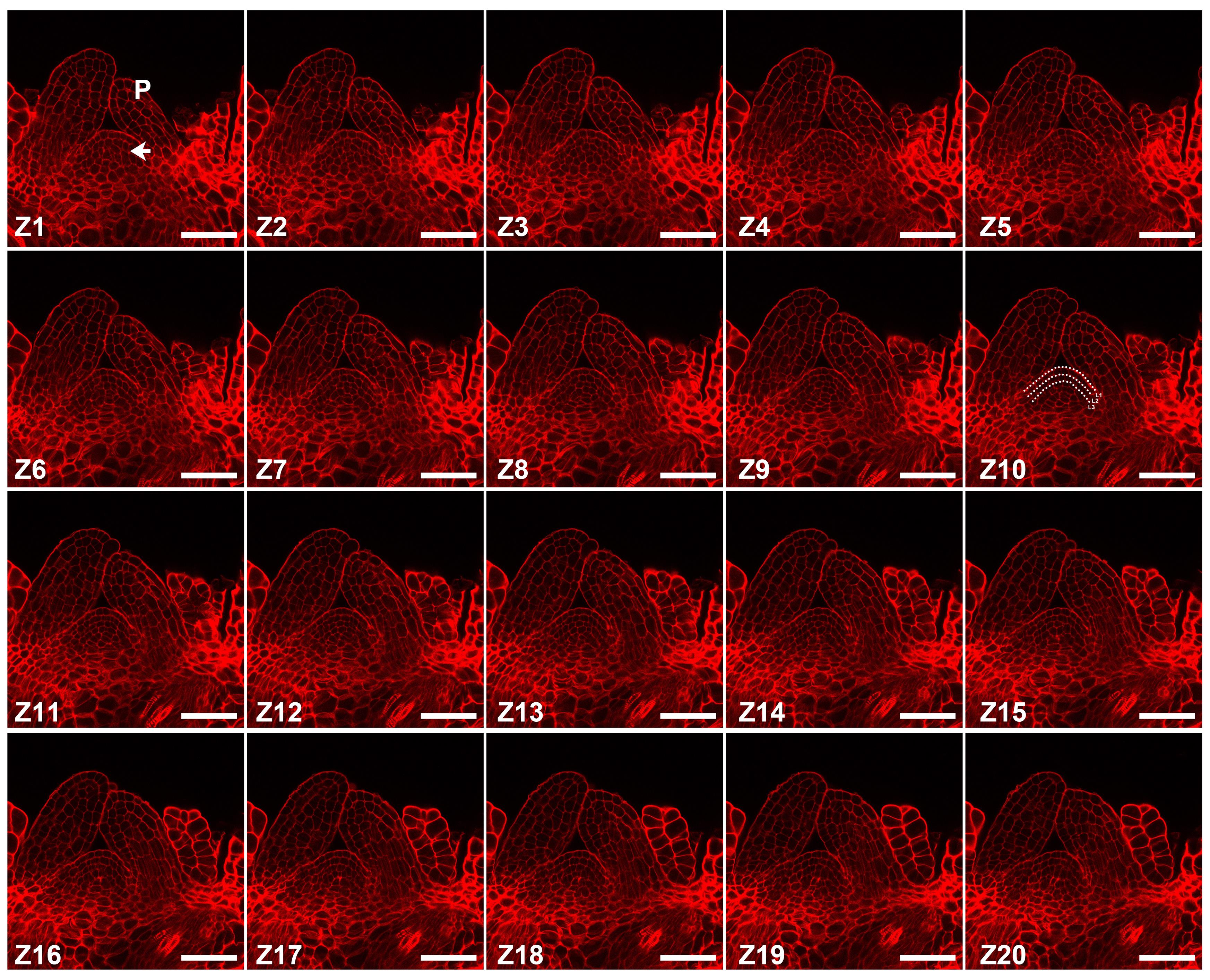
Figure 4. Imaging of shoot tips. Series of SAM images with different z-axis obtained using a laser scanning confocal microscope. The arrow indicates the SAM; P indicates the leaf primordia; Z indicates different z-axis; white dashed lines in Z10 mark the cell layers. Scale bar: 50 μm.
Recipes
Solid MS medium
Dissolve 4.4 g of Murashige and Skoog basal medium and 10 g of sucrose in 1 L of ddH2O. Adjust the pH to 5.8 by adding potassium hydroxide. Then, add 3 g of phytagel and autoclave.
Fixative solution
Add 5 mL of methanol and 1 mL of acetate in 4 mL of ddH2O. The solution should be freshly prepared just before use.
1% periodic acid solution
Dissolve 0.1 g of periodic acid in 10 mL of ddH2O. The solution should be freshly prepared just before use.
Pseudo-Schiff propidium iodide solution
Dissolve 0.1 g of sodium hydrogen bisulfite and 100 μg of propidium iodide in 4.9 mL of ddH2O. Add 62.5 μL of hydrochloric acid.
This solution should be stored away from light and freshly prepared just before use.
Chloral hydrate solution
Dissolve 8 g of chloral hydrate in 2 mL of ddH2O and add 1 mL of glycerol.
This solution can be stored at room temperature for a long time.
Hoyer’s solution
Dissolve 6 g of gum arabic in 10 mL of ddH2O for one day; then, add 5 mL of glycerol and 40 g of chloral hydrate.
Wait 10 days before using; the solution can be stored away from light for a long time.
Ethanol solutions
All ethanol solutions are prepared with 100% ethanol and ddH2O by volume ratio. The 100% ethanol refers to absolute ethyl alcohol.
Acknowledgments
This work was funded by grants from the National Natural Science Foundation of China. This protocol was modified based on the previous publication (Li et al., 2022). The authors thank the Imaging Center, College of Life Sciences, Capital Normal University, Beijing, China.
Competing interests
The author declares no competing financial interests.
References
- Azizi, P., Rafii, M. Y., Maziah, M., Abdullah, S. N., Hanafi, M. M., Latif, M. A., Rashid, A. A. and Sahebi, M. (2015). Understanding the shoot apical meristem regulation: a study of the phytohormones, auxin and cytokinin, in rice. Mech Dev 135: 1-15.
- Balkunde, R., Kitagawa, M., Xu, X. M., Wang, J. and Jackson, D. (2017). SHOOT MERISTEMLESS trafficking controls axillary meristem formation, meristem size and organ boundaries in Arabidopsis. Plant J 90: 435-446.
- Chung, Y., Zhu, Y., Wu, M. F., Simonini, S., Kuhn, A., Armenta-Medina, A., Jin, R., Ostergaard, L., Gillmor, C. S. and Wagner, D. (2019). Auxin response factors promote organogenesis by chromatin-mediated repression of the pluripotency gene SHOOTMERISTEMLESS. Nat Commun 10: 886.
- Dinneny, J. R. and Benfey, P. (2008). Plant stem cell niches: standing the test of time. Cell 132: 553-557.
- Fletcher, J. C. (2018). The CLV-WUS stem cell signaling pathway: a roadmap to crop yield optimization. Plants (Basel) 7(4):87.
- Haseloff, J. (2003). Old botanical techniques for new microscopes. Biotechniques 34: 1174-1178, 1180, 1182.
- Hu, C., Zhu, Y., Cui, Y., Cheng, K., Liang, W., Wei, Z., Zhu, M., Yin, H., Zeng, L., Xiao, Y., et al. (2018). A group of receptor kinases are essential for CLAVATA signalling to maintain stem cell homeostasis. Nat Plants 4: 205-211.
- Koi, S. and Kato, M. (2007). Developmental morphology of the shoot in Weddellina squamulosa and implications for shoot evolution in the Podostemaceae.Ann Bot 99(6):1121-1130.
- Li, R., Wei, Z., Li, Y., Shang, X., Cao, Y., Duan, L. and Ma, L. (2022). SKI-INTERACTING PROTEIN interacts with SHOOT MERISTEMLESS to regulate shoot apical meristem formation. Plant Physiol 189(4):2193-2209.
- Long, J. A., Moan, E. I., Medford, J. I. and Barton, M. K. (1996). A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379: 66-69.
- Mayer, K.F., Schoof, H., Haecker, A., Lenhard, M., Jürgens, G. and Laux, T. (1998). Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805-815.
- Moreno, N., Bougourd, S., Haseloff, J. and Feijo, J. (2006). Imaging plant cells. In: Pawley, J. (Ed.). Handbook of Biological Confocal Microscopy (pp. 769-787). New York: SpringerScience and Business Media.
- Prunet, N., Jack, T. P. and Meyerowitz, E. M. (2016). Live confocal imaging of Arabidopsis flower buds. Dev Biol 419(1): 114-120.
- Shi, B., Zhang, C., Tian, C., Wang, J., Wang, Q., Xu, T., Xu, Y., Ohno, C., Sablowski, R., Heisler, M. G., et al. (2016). Two-step regulation of a meristematic cell population acting in shoot branching in Arabidopsis. PLoS Genet 12(7): e1006168.
- Spinelli, S. V., Martin, A. P., Viola, I. L., Gonzalez, D. H. and Palatnik, J. F. (2011). A mechanistic link between STM and CUC1 during Arabidopsis development. Plant Physiol 156: 1894-1904.
- Steeves, T. A. and Sussex, I. M. (1989). Patterns in plant development (New York: Cambridge University Press).
- Truernit, E., Bauby, H., Dubreucq, B., Grandjean, O., Runions, J., Barthelemy, J. and Palauqui J. C. (2008). High-resolution whole-mount imaging of three-dimensional tissue organization and gene expression enables the study of phloem development and structure in Arabidopsis. Plant Cell 20: 1494-1503.
- Wang, W., Hu, C., Li, X. N., Zhu, Y. F., Tao, L., Cui, Y. W., Deng, D. Q., Fan, X. X., Zhang, H., Li, J., Gou, X. P. and Yi, J. (2022). Receptor-like cytoplasmic kinases PBL34/35/36 are required for CLE peptide-mediated signaling to maintain shoot apical meristem and root apical meristem homeostasis in Arabidopsis.Plant Cell 34(4):1289-1307.
- Williams, L.E. (2021). Genetics of shoot meristem and shoot regeneration. Annu Rev Genet 55: 661-681.
- Zhang, L., DeGennaro, D., Lin, G., Chai, J. and Shpak, E.D. (2021). ERECTA family signaling constrains CLAVATA3 and WUSCHEL to the center of the shoot apical meristem.Development 148(5):dev189753.
- Zhao, Z., Andersen, S. U., Ljung, K., Dolezal, K., Miotk, A., Schultheiss, S. J. and Lohmann, J. U. (2010). Hormonal control of the shoot stem-cell niche. Nature 465: 1089-1092.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Li, R., Li, Q. and Ma, L. (2023). Modified Pseudo-Schiff Propidium Iodide for Staining the Shoot Apical Meristem in Arabidopsis. Bio-protocol 13(9): e4672. DOI: 10.21769/BioProtoc.4672.
Category
Plant Science > Plant developmental biology > Morphogenesis
Developmental Biology > Morphogenesis > Cell structure
Cell Biology > Cell structure > Nucleus
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








