- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Anti-tumor Efficacy of CD19 CAR-T in a Raji B Cell Xenografted Mouse Model
Published: Vol 13, Iss 8, Apr 20, 2023 DOI: 10.21769/BioProtoc.4655 Views: 3429
Reviewed by: Aleksei TikhonovShengwen Calvin LiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
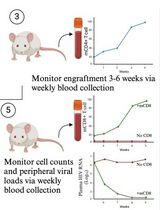
A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2512 Views
Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2532 Views
PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3966 Views
Abstract
Chimeric antigen receptor (CAR)-T therapy launched a new era for cancer treatments, displaying outstanding effectiveness in relapsed or refractory B-cell malignancies. Demonstrating the tumor-killing ability of CAR-Ts in mouse xenograft models serves as a golden criterium in preclinical research. Here, we describe a detailed method for evaluating CAR-T’s function in immune-deficient mice bearing Raji B cell–induced tumors. It includes generating CD19 CAR-T cells from healthy donors, injecting tumor cells and CAR-T cells into mice, and monitoring tumor growth and CAR-T state. This protocol provides a practical guide to evaluate CAR-T’s function in vivo within eight weeks.
Graphical overview

Background
Chimeric antigen receptor (CAR)-T-cell therapy exploits an antibody-dependent, major histocompatibility complex–independent antigen recognition paradigm to specifically target cancer cells using genetically engineered T cells. It is one of the most sought-after tools for treating hematologic malignancies (Daniyan and Brentjens, 2016; Posey et al., 2016). Recently, extensive studies have revealed the molecular mechanisms of CAR-T activation, such as charged lipids and ions–regulated CD28 conformation and signaling (Yang et al., 2017) and LAT-independent formation of CAR microclusters (Dong et al., 2020); SILAC-based phosphoproteomics revealed unique signaling circuits of CAR-Ts, and the distance of synapse from CAR-T/tumor cells was found to affect T-cell function (Xiao et al., 2022). Understanding CAR-T signaling further paved the way to develop new strategies to improve its function (Davenport et al., 2018; Majzner et al., 2020).
Generating CAR-Ts of high quality is a crucial step for validating their function in vitro and in vivo. Virus-based gene delivery systems are commonly used and can achieve high transduction efficiencies (Vormittag et al., 2018). Lentiviral and gamma-retroviral vectors are the most frequently used viral vector systems (Vannucci et al., 2013). Compared to retrovirus, which only infect proliferating cells, lentivirus could infect both proliferating and non-proliferating cells. Thus, lentivirus can infect a broader range of cell types than retroviruses. Meanwhile, lentiviruses need regulatory genes to neutralize the host's anti-viral response and regulate viral replication (Vannucci et al., 2013). They also require a large amount of plasmid for transient transfection to produce a high titer of lentivirus (Merten et al., 2016).
Immunodeficient mice (e.g., NSG, NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJl) are widely used to study the interactions between the human immune system and cancer. They offer a low-barrier platform for evaluating immunotherapeutic effects in the context of human immune cells and tumors (Shultz et al., 2014). Although some of those immunodeficient aged mice develop T and B cells, which causes the leakiness of immune cells (Allen et al., 2019), these mice are the gold standard host for engraftment of human tumors or for establishing human immune components following a hematopoietic stem cell transplant. Furthermore, they allow precise preclinical evaluations of antibody-based therapeutics, cancer vaccines, checkpoint inhibitor therapies, and adoptive cancer immunotherapies (Shultz et al., 2014). NSG mice xenografted with Raji (lymphoma) tumor cells are among the most used and recognized models for evaluating CAR-Ts' efficacy and toxicity targeting CD19 (Pegram et al., 2015; Majzner et al., 2020).
Here, we provide a detailed step-by-step protocol describing how to evaluate CD19 CAR-Ts tumor cell–killing effect in vivo. The protocol covers the generation of CD19 CAR-T cells from healthy donors, injection of tumor cells and CAR-T cells into mice, and monitoring of tumor growth and CAR-T state.
Materials and Reagents
T-25 or T-75 flask (Thermo Fisher, catalog number: 156499 or 156367)
15 mL conical centrifuge tubes (Thermo Fisher, catalog number: 14-959-53A)
50 mL conical centrifuge tubes (Thermo Fisher, catalog number: 14-959-49A)
Non-tissue culture–treated 24-well plates (Sigma, catalog number: CLS3738)
Tissue culture–treated 24-well or 6-well plates (StemCell, catalog number: 38017 or 38016)
Plastic wrap (GLAD Flex’N Seal Food Storage Plastic Bag)
1.5 mL polypropylene microcentrifuge tubes (Fisher Scientific, catalog number: 07-000-243)
5 mL polypropylene snaplock microcentrifuge tube (Fisher Scientific, catalog number: 14-568-101)
5 mL disposable pipettes (VWR, catalog number: 76201-710)
VWR individual tubes (FACS tube) (VWR, catalog number: 83009678)
1 mL syringe (Covidien, catalog number: 1180125158)
Alcohol prep pad (Dukal, catalog number: 853)
Blades (VWR, catalog number: 55411-050)
Micro-hematocrit capillary tubes (Thermo Fisher, catalog number: 22-363-566)
0.22 µm filter (Sigma Millipore, catalog number: GSWP04700)
NSG mice (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJl) (The Jackson Laboratory, Strain #005557)
Cell line: Raji B cell (ATCC, catalog number: CCL-86)
Human peripheral blood mononuclear cells (PBMCs), FRESH (Zenbio, #SER-PBMC-200)
CD19 CAR constructs: pHR-SP-CD19 ScFv (FMC63) – myc-CD8 Stalk-CD8 (CD28-41BB-CD3) cyto-sfGFP (Su lab, plasmid #XSB848) (Xiao et al., 2022)
Lentiviral packaging plasmids (second generation): pMD2.G and psPAX2 (Addgene, #12259 and #12260)
Polybrene (Sigma, catalog number: H9268)
OKT-3 antibody (Thermo Fisher, catalog number: 11161D)
Recombinant human IL-2 (PeproTech, catalog number: 200-02)
1× Dulbecco’s phosphate buffered saline (DPBS) (Thermo Fisher, catalog number: 10010023)
GibcoTM HEPES (1 M) (Thermo Fisher, catalog number: 15-630-080)
1 mg/mL retronectin (Takara, catalog number: T100A/B, stock solution in DPBS)
BSA (Sigma, catalog number: A7030)
AIMV (Thermo Fisher, catalog number: 12055083)
RPMI 1640 (Thermo Fisher, catalog number: 11875093)
Human serum (GemCell, catalog number: 100-512)
Penicillin-Streptomycin-Glutamine (PSG) (Life Tech, catalog number: MT30009CI)
Heat-inactivated fetal bovine serum (FBS) (Life Tech, catalog number: 16140071)
PSG (Life Tech, catalog number: MT30009CI)
EDTA (Thermo Fisher, catalog number: 15-575-020)
Human TruStain FcXTM (Fc receptor blocking solution) (BioLegend, catalog number: 4223020)
Anti-human CD3 antibody (BD Biosciences, catalog number: 558117)
Anti-human CD8 antibody (BioLegend, catalog number: 344750)
Anti-human CD4 antibody (BioLegend, catalog number: 300508)
Anti-human CD279 (PD-1) antibody (BioLegend, catalog number: 621608)
Anti-human CD366 (Tim-3) antibody (BioLegend, catalog number: 345012)
Anti-human CD223 (LAG-3) antibody (BioLegend, catalog number: 345012)
Monoclonal Anti-FMC63 scFv antibody (ACROBiosystems, catalog number: FM3-Y45)
Red blood cell lysing buffer (Sigma, catalog number: R7757-100ML)
UltraComp eBeads Plus compensation beads (Thermo Fisher, catalog number: 01-3333-42)
Matrigel (VWR, catalog number: 47743-716)
DPBS/2% BSA (see Recipes)
T-cell culture media (T-cell media) (see Recipes)
Cell culture media for Raji B cells (Raji media) (see Recipes)
FACS staining buffer (see Recipes)
Equipment
Water bath (Thermo Scientific Precision, model: UX-12150-00)
Cell culture incubator, 37 °C with 5% CO2 (Thermo Fisher, catalog number: 51033776)
Flow cytometer analyzer (BD, LSR II Flow Cytometer or Thermo Fisher, Attune Flow Cytometers)
Centrifuge with a swinging rotor and plate holder (Thermo Fisher, catalog number: 75009210)
Digital Electronic Caliper (Fine Science Tools, catalog number: 30087-00)
EasySepTM Magnet (StemCell, catalog number: 18000)
Automated Cell Counters (Thermo Fisher, model: Countess 3)
Software
FlowJo software (BD, https://www.flowjo.com/solutions/flowjo)
Prism software (GraphPad by Dotmatics, https://www.graphpad.com/)
Procedure
Generation of CD19 CAR-T cells
Prepare T-cell media as in Recipes. Store the medium at 4 °C and warm it to 37 °C before usage.
Note: The T-cell medium should be used within two months.
(Day 1) Count donor PBMC number. Plate 7.5 × 106 cells per well of a 24-well plate in 2 mL of T-cell media containing 300 IU/mL of IL2 and 50 ng/mL of OKT-3.
Note: OKT-3 antibody could be replaced by DynabeadsTM Human T-Activator CD3/CD28 (Thermo Fisher, #11161D or equivalent). Prepare the beads according to the user manual.
(Day 2) Coat non-tissue culture–treated 6-well plates with retronectin at a final concentration of 20 µg/mL in 1.5 mL of DPBS per well. Wrap the plate with plastic wrap and incubate the plate overnight at 4 °C.
Note: Retronectin incubation could be done at 37 °C in a 5% CO2 incubator for 4–6 h. Typically, two 6-well plates (12 wells, 18 mL of retronectin buffer) will be used for each CAR-T transduction.
(Day 3) Warm up centrifuge to 32 °C.
Remove retronectin solution from the 6-well plate using 5 mL disposable pipettes, and then block the plate with 2 mL of DPBS/2% BSA for 30 min at room temperature.
Thaw a lentivirus stock on ice.
Wash each well with 2 mL of DPBS and leave DPBS in the well of the retronectin-coated plates.
Calculate the volume of lentivirus for transduction based on T-cell number (0.5–1 million) and multiplicity of infection (MOI) (10–50).
Note: MOI could be adjusted (5–100) based on your virus titer and total amount. When transducing T cells with lentivirus, the MOI should be calculated. In general, the protocol for the viral transduction must be optimized for each virus independently. The presented step-by-step instruction offers a starting point.
Here is an example:
- Number of T cells to be transduced: 106 cells
- Viral titer: 2 × 108 TU/mL
- MOI: 20
Example:
(Number of cells/viral titer) × MOI = Volume of viral vector
(1 × 106 cells/2 × 108 TU/mL) × 20 = 100 μL
Add the above calculated amount of lentivirus to the T-cell culture medium. Then, remove DPBS from the plate and add 2 mL/well of viral medium.
Wrap the plate with plastic wrap and spin the plate at 2,000 × g for 2 h at 32 °C.
During the spin, harvest, wash, and count T cells to be transduced.
Note: It is recommended to include a negative control of un-transduced wells and a positive control (e.g., GFP) of transduced wells.
When the centrifugation of plates is finished, remove the viral medium.
Add 0.5 × 106–1 × 106 T cells per well in the T-cell culture media (300 IU/mL of IL-2) at the concentration of 0.25 × 106 cells per well containing 8 µg/mL polybrene.
Wrap the plate and spin at 500 × g for 10 min with the acceleration and brake set to the lowest setting at 32 °C.
Gently remove the plate from the centrifuge and leave it in the incubator overnight.
(Day 4) Transfer transduced cells onto a new tissue culture–treated 6-well plate to get rid of retronectin. Cells from the identically treated wells can be combined into a T-25 or T-75 flask at a concentration of 0.5 × 106 cells per well.
Verify CAR-GFP expression using fluorescence microscopy, if available (see Figure 1 for representative data).
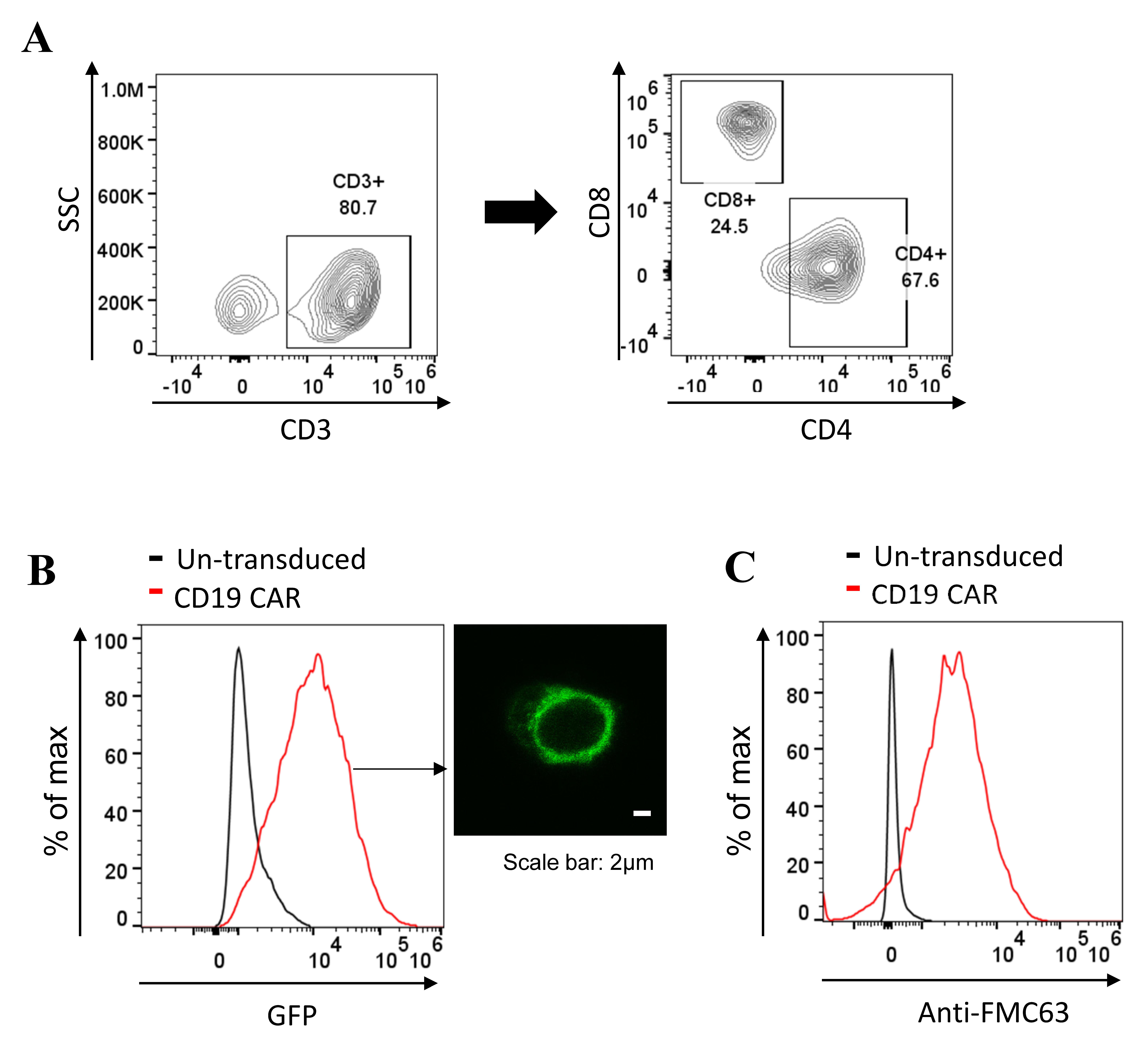
Figure 1. Detection of T-cell population (A), CAR expression by GFP (B), and anti-FMC63 antibody (C). Un-transduced T cells and CD19 CAR-T cells were pre-gated on the CD3+ population; CD4+ and CD8+ were gated from CD3+; CAR-T cells were gated based on GFP expression or anti-FMC63 staining. Data were first published in Xiao et al. (2022) and presented in a modified way.(Day 6) Perform flow cytometry analysis of CAR-GFP expression, three days post-transduction (see Procedure B) (see Figure 1 for representative data).
Continue to inspect T-cell culture daily. Cell numbers were calculated using an Automated Cell Counter. Split culture when cells reach a density of 1 × 106 cells/mL.
Note: A 9–12-day expansion period should be adequate to obtain enough CAR-T cells for mouse injection. Upon stimulation with DynabeadsTM, expression of CD3 will be transiently downregulated, which might affect the staining of CD3 on CAR-T.
Flow cytometry analysis of CAR-GFP expression
Count the cell number of transduced CD19 CAR-T cells and un-transduced T cells. Spin down 0.5 million cells for 5 min (300–400 × g) at 4 °C and discard the supernatant.
Resuspend the cell pellet in FACS staining buffer and centrifuge cells as in Step B1. Then, resuspend the pellet in 50 μL of FACS staining buffer to reach a final cell density of 1 × 107/mL.
[Optional] Block non-specific Fc-mediated interactions: pre-incubate the cells with 5 μL of human TruStain FcXTM per 100 μL for 5–10 min on ice before staining.
Add the recommended amount of fluorochrome-labeled antibody to cells and incubate in the dark for 30 min on ice (e.g., 2.5 μL of anti-human CD3 antibody, 2.5 μL of anti-human CD8 antibody, and 2.5 μL of anti-human CD4 antibody for 0.5 million cells in 100 μL of FACS staining buffer).
Note: Higher temperatures and/or a longer incubation time may lead to non-specific cell labeling.
Staining performed on ice requires increased incubation times.
Add 1 mL of FACS staining buffer to each 1.5 mL Eppendorf tube. Centrifuge samples at 300–400 × g for 5 min at room temperature and then discard the supernatant.
Resuspend stained cells in an appropriate volume (400–600 μL) of FACS staining buffer.
Prepare single-color compensation controls and set up the compensation following the manual (MAN0019374_UltraComp_eBeads_and_UltraComp_eBeads_Plus_Compensation_Beads_PI).
Once the compensation is set up, acquire data on the flow cytometer LSRII or Attune Flow Cytometers.
Note: Cells must be analyzed within 1 h after staining.
The T-cell purity and CAR expression were analyzed by FlowJo software (see Figure 1 for representative data).
Note: We recommend using CAR-T cells with higher transduction (~50%) for in vivo study. Please increase the volume of virus to achieve higher transduction.
In vivo Raji B tumor killing assay
Order 6–7-week-old NSG mice from The Jackson Laboratory. Once the mice are delivered, let them adapt to the local facility for one week before performing tumor cell injection.
Thaw and culture Raji B cells in Raji media (as in Recipes) for 3–5 days before injecting them into mice.
Count Raji cell numbers and centrifuge them at 300–400× g for 10 min at 4 °C. Discard the supernatant.
Resuspend Raji cells with pre-cooled PBS. Centrifuge cell suspension at 300–400 × g for 10 min at 4 °C and discard the supernatant. Repeat once.
Resuspend Raji cells with pre-cooled PBS (1 × 106 Raji cells in 50 µL for each mouse) and transfer the cells into a 1.5 mL Eppendorf tube.
Mix cells with an equal volume of Matrigel (Matrigel is stored in a -80 °C freezer. It needs to be thawed on ice), e.g., 50 µL of cells with 50 µL of Matrigel. This process needs to be performed on ice.
Transfer the mixed cells on ice to the animal facility for tumor injection.
Before injection, pellet mixed cells and avoid bubbles. Take 100 µL of mixture via 1 mL syringe.
Perform a subcutaneous injection. Wet the right flank of mice with an alcohol prep pad and directly inject the cells into the mouse.
Seven days later, prepare for CAR-T cell infusion. The tumor size should be between 20 and 100 mm3 at the time of the T-cell injection.
Count CD19 CAR-T cell number and centrifuge CAR-T cells at 300–400× g for 10 min at 4 °C. Discard the supernatant.
Resuspend CD19 CAR-T cells with pre-cooled PBS and centrifuge cell suspension at 300–400 × g for 10 min at 4 °C; then, discard the supernatant. Repeat once.
Resuspend CD19 CAR-T cells with pre-cooled PBS (1 × 107 cells in 100 µL for each mouse) and transfer cells into a 1.5 ml Eppendorf tube.
Infuse 1 × 107 CD19 CAR-T cells in 100 µL of PBS to tumor-bearing NSG mice intravenously; the control mice will be treated with 100 µL of PBS via tail-vein injection.
Monitor mice's body weight and tumor size every other day. Measure the tumor size using a caliper. Tumor volume is calculated with the formula: V = ½ (length × width2) (see Figure 2 for representative data).
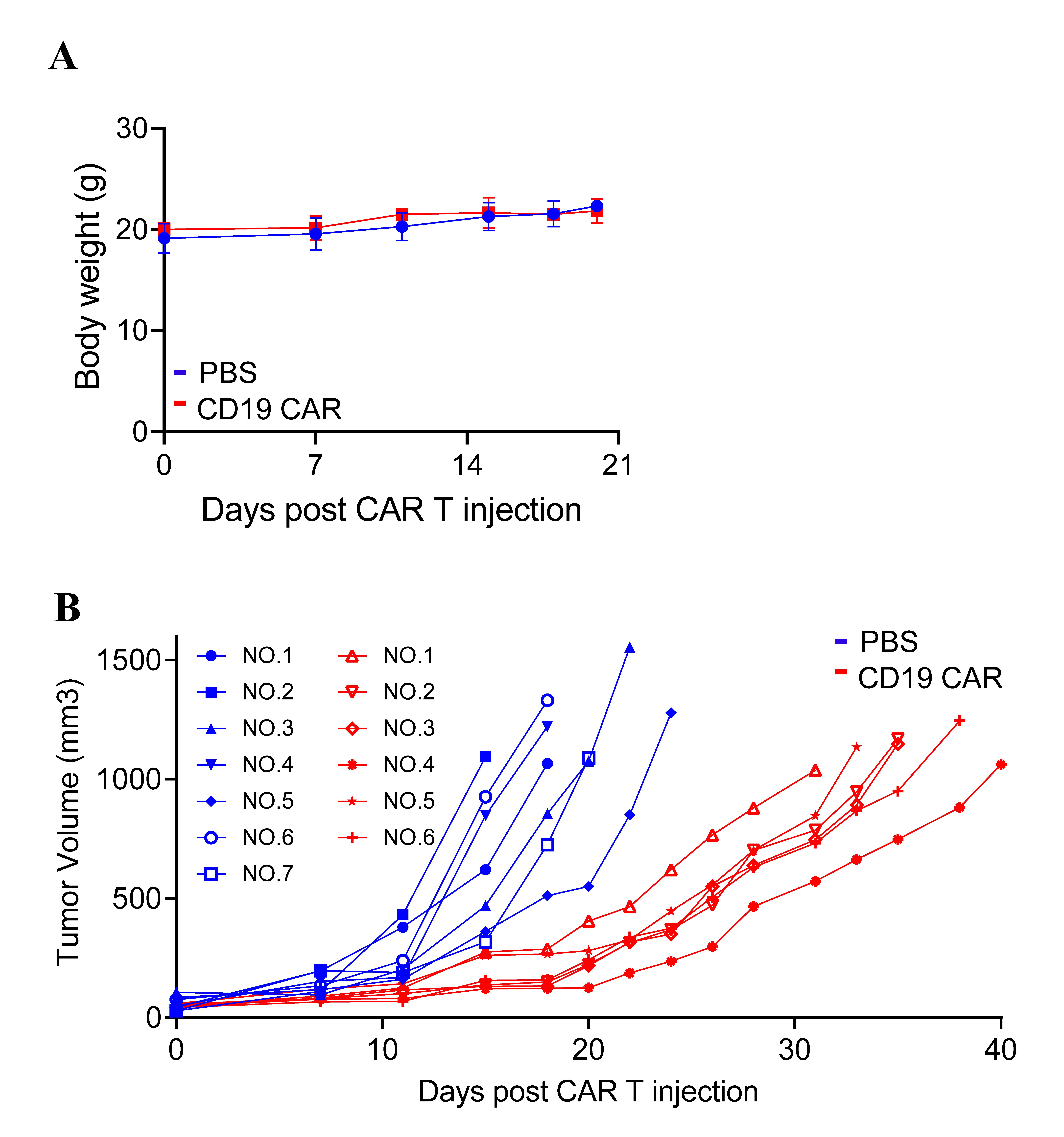
Figure 2. Monitoring body weight (A) and tumor size (B) in PBS-treated and CD19 CAR-T treated Raji-bearing mice. (A) Mouse body weight was measured after CAR-T cell infusion. (B) Individual tumor growth from the untreated PBS group (blue, seven mice) and CD19 CAR-Ts treated group (red, six mice) is presented. Data were first published in Xiao et al. (2022) and presented in a modified way.On day 7, 14, and 21 post-CAR-T cells infusion, tail blood is taken by blade and capillary to verify CAR-T cell percentage and exhaustion status (see Procedure D) (representative data is shown in Figures 3 and 4).
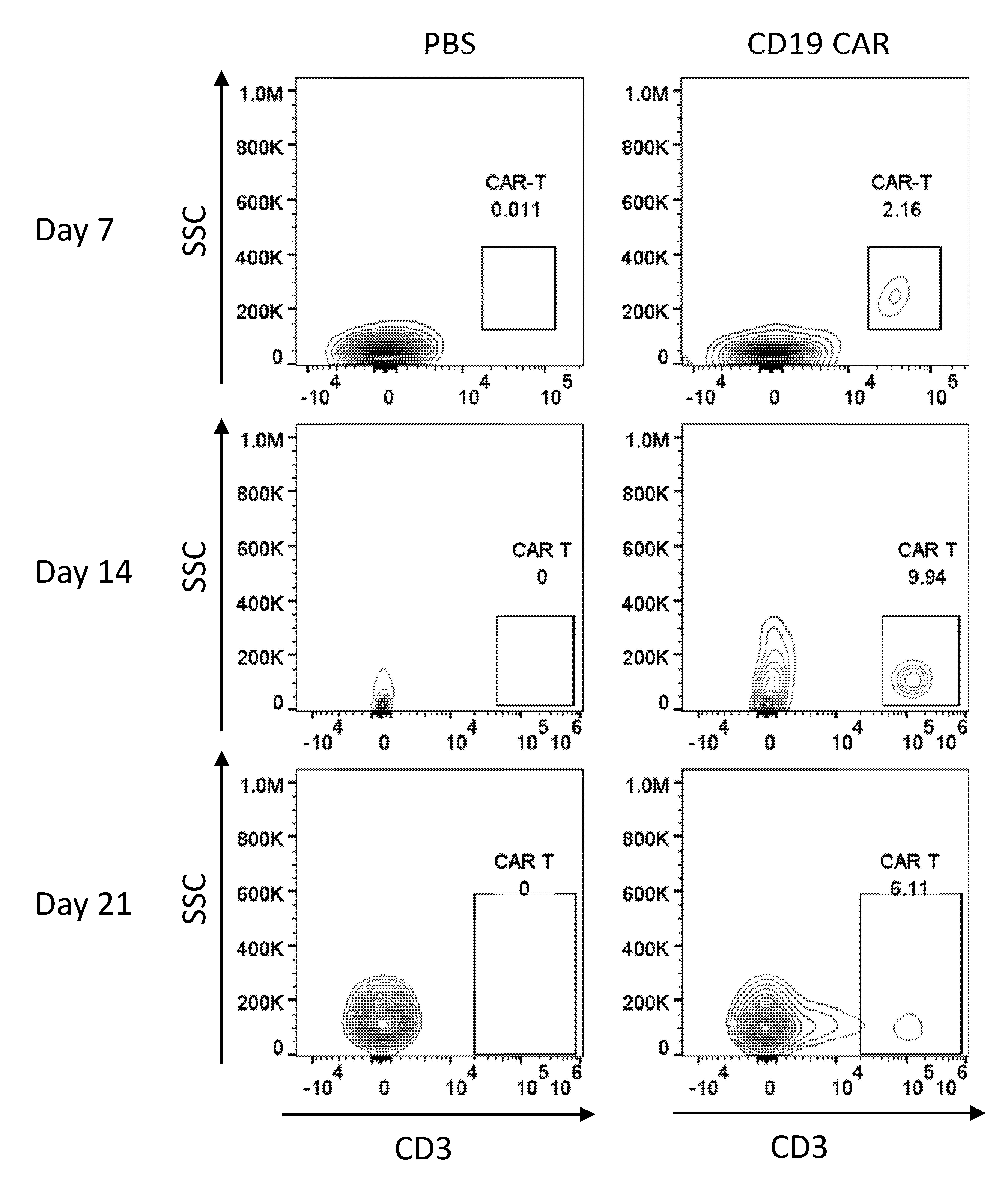
Figure 3. Representative data of CAR-T cell percentage in circulation. On days 7, 14, and 21 after CAR-T infusion, the tail vein blood of mice from untreated and treated groups were harvested to examine CAR-T cell percentage by FACS. The percentage of CAR-Ts in circulation could be used as a parameter to determine CAR-Ts persistence in vivo. Data were first published in Xiao et al. (2022) and presented in a modified way.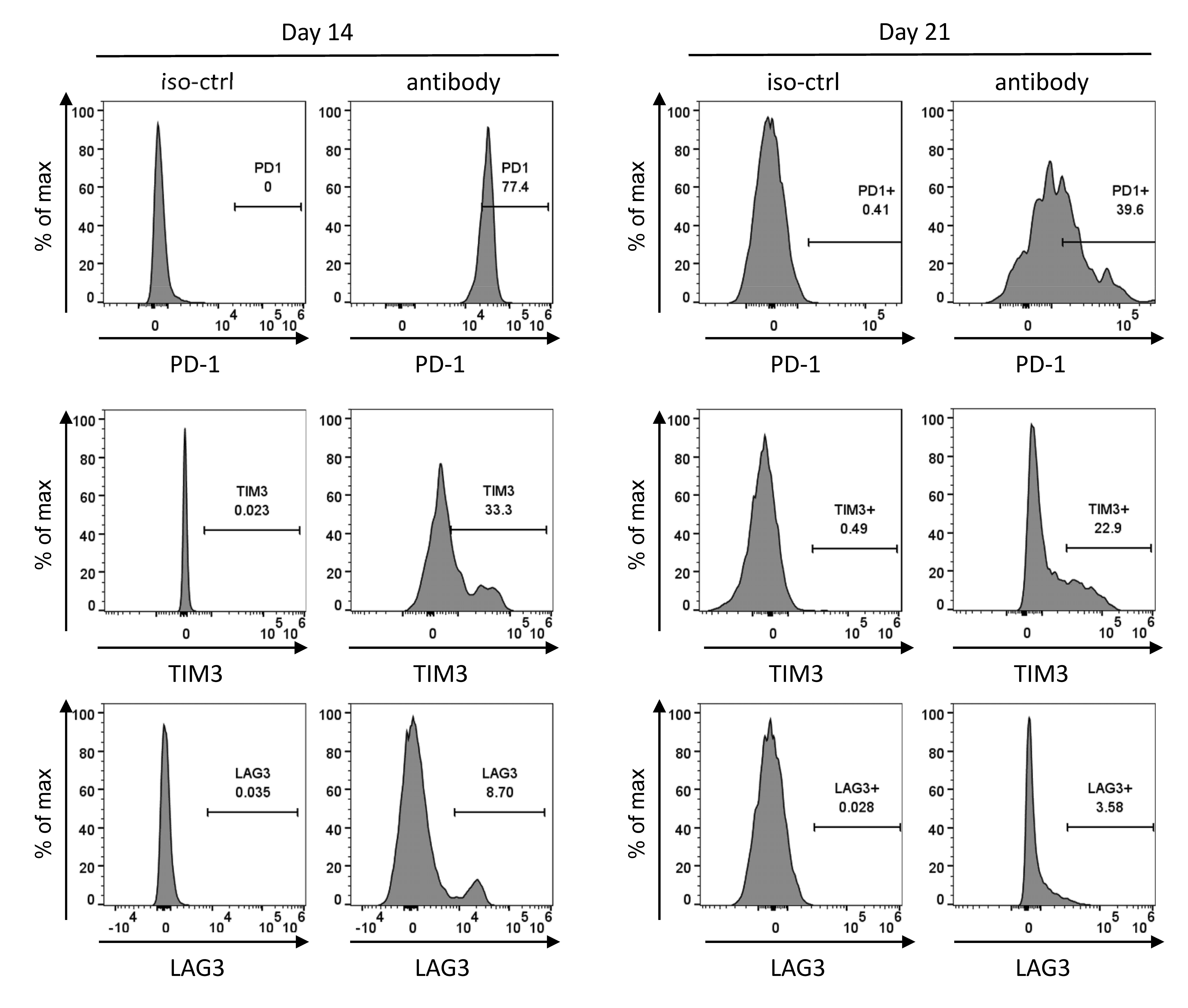
Figure 4. Representative data of T-cell exhaustion markers expressed on CD19 CAR-Ts. On days 14 and 21 after CAR-Ts infusion, the tail vein blood of mice was harvested to examine the expression of T-cell exhaustion markers, including PD-1, TIM-3, and LAG-3 by FACS (isotype antibody served as staining negative control). Data were first published in Xiao et al. (2022) and presented in a modified way.Euthanize mice using CO2 inhalation when tumor size reaches ∼1,500 mm3.
Monitoring CAR-T cell percentage and exhaustion status in vivo
On days 7, 14, and 21 after CAR-T cell infusion, use a sterile scalpel blade to cut the tail. Clip no more than 1 mm of tail tissue and collect blood by gently milking the tail through the capillary.
Stop blood flow by applying pressure with sterile gauze.
Transfer blood to 100 µL of PBS containing 3 mM EDTA in a 1.5 mL Eppendorf tube.
Centrifuge cell suspension at 300–400 × g for 10 min at 4 °C and discard supernatant.
Incubate cells with 0.2 mL of red blood cell lysis buffer at room temperature for 5 min to remove erythrocytes. Top up the tube with 1 mL of FACS staining buffer and pellet cells (300–400 × g) at 4 °C for 5 min.
Repeat Step D5 once or twice if necessary.
Resuspend cells with 100 µL of FACS staining buffer.
Add fluorochrome-labeled antibodies to cells and incubate in the dark for 30 min on ice. Prepare single-color compensation controls and set up the compensation.
Add 1 mL of FACS staining buffer to each sample. Centrifuge samples at 300–400 × g for 5 min at room temperature and discard the supernatant.
Resuspend stained cells in 400–600 µL of FACS staining buffer and acquire data on the flow cytometer LSRII or Attune Flow Cytometers (for representative data see Figures 3 and 4).
Recipes
DPBS/2% BSA
10 g of BSA in 500 mL of DPBS
Filter through a 0.22 µm filterT-cell culture media (T-cell media)
45% AIMV
45% RPMI 1640
10% human serum
1% PSG
2.5% HEPES (1 M)
Cell culture media for Raji B cells (Raji media)
RPMI 1640, 10% heat-inactivated FBS, 1% PSG
FACS staining buffer
DPBS with 2% FBS and 1 mM EDTA
Acknowledgments
X.S. was supported by an American Cancer Society Research Scholar Grant, the Charles H. Hood Foundation Child Health Research Awards, the Andrew McDonough B+ Foundation Research Grant, the Gilead Sciences Research Scholars Program in Hematology/Oncology, the Rally Foundation and Bear Necessities Foundation A Collaborative Pediatric Cancer Research Awards Program, the Rally Young Scholar Program, the Yale SPORE in skin cancer DRP Award CA121974, the Yale DeLuca Pilot Award, the NIGMS MIRA (R35) program GM138299, and the Human Frontier Science Program Early-Career Research Grant. FACS Services, results, and/or products in support of the research project were generated by the Rutgers Cancer Institute of New Jersey Immune Monitoring Shared Resource, supported in part by funding from the NCI-CCSG P30CA072770-5920. This protocol is derived from the original research paper (Xiao et al., 2022; 10.1126/sciimmunol.abl3995).
Competing interests
X.S. is a co-applicant for a provisional patent on CAR, which is not based on the specific results in this manuscript. The other authors declare that they have no competing interests.
Ethics
Mice were housed under specific pathogen–free conditions and cared for in accordance with U.S. National Institutes of Health guidelines, and all procedures were approved by the Rutgers University Animal Care and Use Committee (PROTO202100020).
References
- Allen, T. M., Brehm, M. A., Bridges, S., Ferguson, S., Kumar, P., Mirochnitchenko, O., Palucka, K., Pelanda, R., Sanders-Beer, B., Shultz, L. D., et al. (2019). Humanized immune system mouse models: progress, challenges and opportunities. Nat Immunol 20(7): 770-774.
- Daniyan, A. F. and Brentjens, R. J. (2016). At the Bench: Chimeric antigen receptor (CAR) T cell therapy for the treatment of B cell malignancies. J Leukoc Biol 100(6): 1255-1264.
- Davenport, A. J., Cross, R. S., Watson, K. A., Liao, Y., Shi, W., Prince, H. M., Beavis, P. A., Trapani, J. A., Kershaw, M. H., Ritchie, D. S., et al. (2018). Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc Natl Acad Sci U S A 115(9): E2068-E2076.
- Dong, R., Libby, K. A., Blaeschke, F., Fuchs, W., Marson, A., Vale, R. D. and Su, X. (2020). Rewired signaling network in T cells expressing the chimeric antigen receptor (CAR). EMBO J 39(16): e104730.
- Majzner, R. G., Rietberg, S. P., Sotillo, E., Dong, R., Vachharajani, V. T., Labanieh, L., Myklebust, J. H., Kadapakkam, M., Weber, E. W., Tousley, A. M., et al. (2020). Tuning the Antigen Density Requirement for CAR T-cell Activity. Cancer Discov 10(5): 702-723.
- Merten, O. W., Hebben, M. and Bovolenta, C. (2016). Production of lentiviral vectors. Mol Ther Methods Clin Dev 3: 16017.
- Pegram, H. J., Purdon, T. J., van Leeuwen, D. G., Curran, K. J., Giralt, S. A., Barker, J. N. and Brentjens, R. J. (2015). IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of B-cell acute lymphoblastic leukemia. Leukemia 29(2): 415-422.
- Posey, A. D., Jr., Schwab, R. D., Boesteanu, A. C., Steentoft, C., Mandel, U., Engels, B., Stone, J. D., Madsen, T. D., Schreiber, K., Haines, K. M., et al. (2016). Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 44(6): 1444-1454.
- Shultz, L. D., Goodwin, N., Ishikawa, F., Hosur, V., Lyons, B. L. and Greiner, D. L. (2014). Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb Protoc 2014(7): 694-708.
- Vannucci, L., Lai, M., Chiuppesi, F., Ceccherini-Nelli, L. and Pistello, M. (2013). Viral vectors: a look back and ahead on gene transfer technology. New Microbiol 36(1): 1-22.
- Vormittag, P., Gunn, R., Ghorashian, S. and Veraitch, F. S. (2018). A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol 53: 164-181.
- Xiao, Q., Zhang, X., Tu, L., Cao, J., Hinrichs, C. S. and Su, X. (2022). Size-dependent activation of CAR-T cells. Sci Immunol 7(74): eabl3995.
- Yang, W., Pan, W., Chen, S., Trendel, N., Jiang, S., Xiao, F., Xue, M., Wu, W., Peng, Z., Li, X., et al. (2017). Dynamic regulation of CD28 conformation and signaling by charged lipids and ions. Nat Struct Mol Biol 24(12): 1081-1092.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Xiao, Q. and Su, X. (2023). Anti-tumor Efficacy of CD19 CAR-T in a Raji B Cell Xenografted Mouse Model. Bio-protocol 13(8): e4655. DOI: 10.21769/BioProtoc.4655.
- Xiao, Q., Zhang, X., Tu, L., Cao, J., Hinrichs, C. S. and Su, X. (2022). Size-dependent activation of CAR-T cells. Sci Immunol 7(74): eabl3995.
Category
Immunology > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link