- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Modified Acyl-RAC Method of Isolating Retinal Palmitoyl Proteome for Subsequent Detection through LC-MS/MS
Published: Vol 13, Iss 8, Apr 20, 2023 DOI: 10.21769/BioProtoc.4654 Views: 2628
Reviewed by: Ansul LokdarshiRicardo Urquidi Camacho

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Site-specific Incorporation of Phosphoserine into Recombinant Proteins in Escherichia coli
Phillip Zhu [...] Richard B. Cooley
Nov 5, 2022 3553 Views
Quantification of Proliferating and Mitotically Active Retinal Cells in Mice by Flow Cytometry
Hope K. Vanzo-Sparks [...] Cindy L. Linn
Jul 5, 2024 2274 Views
Preparation of Protein Lysates Using Biorthogonal Chemical Reporters for Click Reaction and in-Gel Fluorescence Analysis
Yaxin Xu and Tao Peng
Nov 20, 2024 2007 Views
Abstract
Palmitoylation is a unique and reversible posttranslational lipid modification (PTM) that plays a critical role in many cellular events, including protein stability, activity, membrane association, and protein–protein interactions. The dynamic nature of palmitoylation dictates the efficient sorting of various retinal proteins to specific subcellular compartments. However, the underlying mechanism through which palmitoylation supports efficient protein trafficking in the retina remains unclear. Recent studies show that palmitoylation can also function as a signaling PTM, underlying epigenetic regulation and homeostasis in the retina. Efficient isolation of retinal palmitoyl proteome will pave the way to a better understanding of the role(s) for palmitoylation in visual function. The standard methods for detecting palmitoylated proteins employ 3H- or 14C-radiolabeled palmitic acid and have many limitations, including poor sensitivity. Relatively recent studies use thiopropyl Sepharose 6B resin, which offers efficient detection of palmitoylated proteome but is now discontinued from the market. Here, we describe a modified acyl resin–assisted capture (Acyl-RAC) method using agarose S3 high-capacity resin to purify palmitoylated proteins from the retina and other tissues, which is greatly compatible with downstream processing by LC-MS/MS. Unlike other palmitoylation assays, the present protocol is easy to perform and cost-effective.
Graphical overview

Background
The retina is a thin layer of neural tissue lining the back of the eye, containing six different cell types organized together for visual perception. Vision is initiated through retinal photoreceptors, which are polarized and compartmentalized neurons containing ciliated outer segments (OS). Photoreceptor OS contains thousands of double membranous discs that harbor phototransduction proteins, which are responsible for capturing light and converting it into an electrical signal. The OS discs, along with the proteins that mediate phototransduction within the ciliated structures, are phagocytized in a circadian rhythm by pigmented epithelial cells that abut the retinal layer. On the basal side of the photoreceptor neurons lay the ribbon synapses that are responsible for the transmission of light-dependent electrical signals to downstream neurons, which ultimately transmit the signals to the visual cortex. The proteins present at the synapse and in the OS disc membranes are synthesized in the inner segment (IS) of the photoreceptor neurons and trafficked to their destination. The constant renewal of the OS discs necessitates continual protein synthesis and efficient sorting machinery (Burns and Arshavsky, 2005; Baker et al., 2008).
The protein trafficking machinery in photoreceptor neurons is greatly aided by the lipid modification of proteins (Kolandaivelu et al., 2009;Maeda et al., 2010;Yang and Wensels, 1992). Among the lipid modifications, palmitoylation is the most common form of protein acylation, which adds a 16-carbon palmitic acid to a cysteine residue in the protein. Unlike most lipid modifications, palmitoylation is unique and reversible, and therefore plays a dynamic role in dictating the localization of a protein both spatially and temporally. Defects in the sorting of proteins due to the absence/loss of palmitoylation have been associated with various inherited blinding diseases, suggesting an important role of this lipid modification. Several studies including ours described the importance of palmitoylation in retinal health and function. We showed that lack of palmitoylation in a retinitis pigmentosa–associated protein—progressive rod-cone degeneration (PRCD)—leads to protein mistargeting to the photoreceptor IS where it undergoes severe destabilization (Murphy and Kolandaivelu, 2016). Likewise, mutating palmitoylation sites in rhodopsin leads to severe light-induced photoreceptor degeneration (Maeda et al., 2010). However, a paucity of information on retinal palmitoylated proteins hinders our progress toward understanding the significance of this crucial modification in retinal structure and function. Our modified acyl resin–assisted capture (Acyl-RAC) method efficiently identifies the global palmitoyl proteome in retinal neurons and other tissues that are compatible with downstream mass spectrometry (LC-MS/MS) analyses for further validation (Forrester et al., 2011;Murphy and Kolandaivelu, 2016; Myers et al., 2022). Using our protocol, we show the efficient isolation of palmitoylated proteins and downstream validation of known palmitoylated proteins by immunoblotting. Using this protocol, we show that several proteins that are critical for proper retinal function, and proteins linked with blinding diseases, are post-translationally lipid-modified by palmitoylation. Additionally, we show that our protocol can be reliably used to identify palmitoylated proteins in other tissues.
Materials and Reagents
Eppendorf 1.5 mL tubes (Thermo Fisher Scientific, catalog number: 05-408-129)
1 M HEPES, pH 7.5 (Thermo Fisher, catalog number: 15630080)
5 M NaCl (Thermo Fisher Scientific, catalog number: 00622643)
500 mM EDTA (Thermo Fisher Scientific, catalog number: AM9260G)
Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: 8170341000)
Hydroxylamine-HCl (Thermo Fisher Scientific, catalog number: 270100010)
Pierce protease inhibitor tablet, EDTA free (Thermo Fisher Scientific, catalog number: A32955)
Phosphatase inhibitor (Thermo Fisher Scientific, catalog number: J63907.AA)
HDSF (Santa Cruz, catalog number: sc-221708A)
Methyl methanethiosulfonate (MMTS) (Sigma-Aldrich, catalog number: 2949920)
100% acetone (Thermo Fisher Scientific, catalog number: 186947)
2-Mercaptoethanol (β-ME) (Calbiochem, catalog number: UN2966)
1 M dithiothreitol (DTT) (Sigma-Aldrich, catalog number: D0632)
Agarose S3 high-capacity acyl-RAC capture resin (Nanocs, catalog number: AR-SS-2)
0.5 M Tris-HCl, pH 6.8 (Thermo Fisher Scientific, catalog number: J63735.K2)
100% glycerol (Thermo Fisher Scientific, catalog number: 15548224)
Bromophenol blue (Sigma-Aldrich, catalog number: B5525)
3 kD cutoff centrifugal filter (Millipore, catalog number: Z677094)
Trichloroacetic acid (TCA) (Thermo Fisher Scientific, catalog number:184151000)
Coomassie stain (Bio-Rad, catalog number:1610786)
Triton X-100 (Sigma-Aldrich, catalog number: 9036195)
Sodium hydroxide (NaOH) (Thermo Fisher Scientific, catalog number: A16037.36)
Lysis buffer base (see Recipes)
Lysis buffer – working solution (see Recipes)
Binding buffer (see Recipes)
Blocking buffer (see Recipes)
5× Laemmli sample buffer (see Recipes)
1× Laemmli sample buffer (see Recipes)
1 M dithiothreitol (DTT) (see Recipes)
100% TCA stock preparation (w/v) (see Recipes)
Tris-glycine SDS running buffer (Thermo Fisher Scientific, catalog number: LC26755) (see Recipes)
70% acetone (see Recipes)
2 M hydroxylamine (see Recipes)
2 M sodium chloride (see Recipes)
5 M sodium hydroxide (See Recipes)
10% Triton X-100 (see Recipes)
Equipment
Sonicator (Fisher, Microson Ultrasonic cell disruptor, model: CL-18)
Heat block (Thermo Fisher Scientific, catalog number: 11715125D)
Vortex (Scientific Industries, model: G-560)
Centrifuge, (Eppendorf, catalog number: 0011627)
Nutator (Thermo Fisher Scientific, model: 88881001)
Software
Excel 2016 (Microsoft)
GraphPad Prism (Version 9.0)
Adobe Illustrator 2023
CSS-Palm 4.0 – Palmitoylation Sites Prediction tool (Ren et al., 2008)
Procedure
Tissue lysis
Take four freshly collected or flash-frozen mouse retinae in a 1.5 mL Eppendorf tube and add 300 μL of lysis buffer (see Recipes) (Murphy and Kolandaivelu, 2016).
Sonicate the tissue at 12–15 psi thrice, four seconds each, with 30 s of incubation on ice between pulses.
To solubilize the proteins, add 33 µL of 10% Triton X-100 (to a final concentration of 1% Triton X-100) and nutate the samples at 4 °C for 15 min.
Remove cell and nuclear debris by centrifuging at 200 × g and 4 °C for 3 min and transfer the supernatant into a fresh 1.5 mL tube.
Blocking free thiol groups
Prepare blocking buffer containing MMTS (add 4 µL of MMTS per every 300 µL of blocking buffer).
Separate supernatant from step A4 into 100 µL aliquots (in 1.5 mL tubes) and add three volumes of blocking buffer containing MMTS (300 µL).
Note: Briefly vortex the reaction mixture before each use to keep the solution homogeneous.
Incubate samples in the heat block at 40 °C for 16 min with a 5 s vortex every 2 min.
Protein precipitation
Add 1.1 mL of 100% ice-cold acetone into each tube, invert thrice, and incubate at -20 °C for 20 min.
Centrifuge samples at 20,000 × g and 4 °C for 15 min to pellet the proteins.
Discard the supernatant and wash the protein pellet four times with ice-cold 70% acetone by gentle vortexing followed by centrifugation at top speed for 1 min at 4 °C.
Note: Dislodge the pellet during the first wash to remove the residual MMTS between the pellet and the Eppendorf tube wall.
Cleaving thioester bonds with hydroxylamine (HA) & capturing palmitoylated proteins using agarose S3 high-capacity acyl-RAC resin
Air-dry the protein pellet at room temperature (RT) for ~30 min to remove residual acetone.
Note: Do not over-dry the protein pellet to avoid issues with resuspension.
Resuspend each pellet in 200 µL of binding buffer by brief sonication and pool samples into one tube (e.g., 200 µL × three tubes = 600 µL).
Pipette 40 µL of agarose S3 high-capacity resin into each of the two clean 1.5 mL Eppendorf tubes labeled as Elute + HA (E +HA) and Elute - HA (E -HA) and wash to equilibrate the resin with 500 µL of binding buffer once. Allow the beads to pellet by gravity and discard the wash buffer.
Note: Make sure the resin is homogeneously resuspended before use.
Pipette 75 µL of the pooled sample to a clean 1.5 mL Eppendorf tube labeled as Total.
Add 250 µL of sample to each of the E +HA and E -HA tubes (if there is more sample volume, add additional beads accordingly and split equal sample volume for +HA and -HA; if there is less sample volume, make it up to 600 µL with elution buffer).
Add 45 µL of 2 M hydroxylamine to the E +HA tube and add 45 µL of 2 M sodium chloride to the E -HA tube.
Nutate all samples, including the total fraction sample at RT for 2 h.
Let the beads fall by gravity and collect 75 µL of the supernatant from each of the E +HA and E-HA tubes into clean 1.5 mL Eppendorf tubes labeled as Unbound +HA (UB +HA) and Unbound -HA (UB -HA), respectively.
Elution of bound protein
Wash E +HA and E -HA beads by adding 1 mL of binding buffer and gently inverting the tubes approximately six times. Let the beads fall by gravity and discard the supernatant. Repeat the wash step three more times.
Add 75 µL of binding buffer containing 10 mM DTT to each of the E +HA and E -HA tubes and incubate at RT for 10 min to elute the bound proteins.
Note: To make the above elution buffer, add 10 µL of 1M DTT in 990 µL of binding buffer and mix thoroughly by vortexing. Scale up as needed.
Repeat the elution step 2–3 times and pool the eluates.
Note: The majority (90%) of proteins are eluted in the first fraction (i.e., elute 1). Check each eluted fraction by western blotting with known palmitoylated proteins, e.g., Rhodopsin, Goα, and PRCD. If the second and third elute show significant amounts of known palmitoylated proteins, pool the eluates and use TCA precipitation or suitable Microcon cutoff filters to concentrate the samples.
To concentrate the samples, transfer the pooled eluates to Pierce Concentrator 3K MWCO filter and centrifuge at 6,000–9,000 × g for 30 min at RT.
Notes:
Samples can be stored at -20 °C for approximately one month until further processing.
Optional: Eluates can be further concentrated by using TCA precipitation. See the protocol below.
TCA precipitationAdd one volume of TCA [50 µL of 100% TCA stock preparation (w/v)] to four volumes of protein samples (200 µL of eluted protein samples) and incubate the tubes for 15 min on ice.
Centrifuge the tubes at 18,400 × g and 4 °C for 5 min to pellet the proteins.
Carefully remove the supernatant, leaving the protein pellet intact.
Wash the pellet with 200 µL of ice-cold acetone and centrifuge the tube at 18,500 × g and 4 °C for 5 min. Discard the supernatant and repeat the wash step.
Air-dry the pellet for 5–10 min to remove residual acetone.
Identification of palmitoylated protein by SDS-PAGE/ LC-MS/MS
To perform immunoblotting, add 18.75 µL of 5× Laemmli sample buffer containing a final concentration of 5% 2-mercaptoethanol (β-ME) and 10 mM DTT to 75 µL of Total and Unbound samples.
Add 70 µL of 1× Laemmli sample buffer containing 5% β-ME and 10 mM DTT to both +HA and -HA eluted fractions.
Let the tubes sit at RT for 20 min with intermittent mixing every 5 min.
Boil the samples for 5 min on a floating rack in the water bath and centrifuge at 6,000 × g for 30 s at RT.
To detect palmitoylated proteins by mass spectrometry, run the samples on a 12% SDS-PAGE gel at 80 V for 30 min (Figures 1 and 2).
Note: Ensure the electrophoresis equipment is thoroughly cleaned with ddH2O and use fresh SDS-PAGE buffer.
Transfer the gel onto a clean glass tray and perform Coomassie staining (Figure 3A).
After overnight destaining with Milli-Q water (autoclaved), cut the protein bands to perform in-gel digestion (see Figure 3A) and process the samples for LC-MS/MS analysis (Edmonds et al., 2017)
For additional validation, perform immunoblotting and probe the blots with antibodies against the proteins of interest to identify/show their palmitoylation status (Figures 1 and 2).
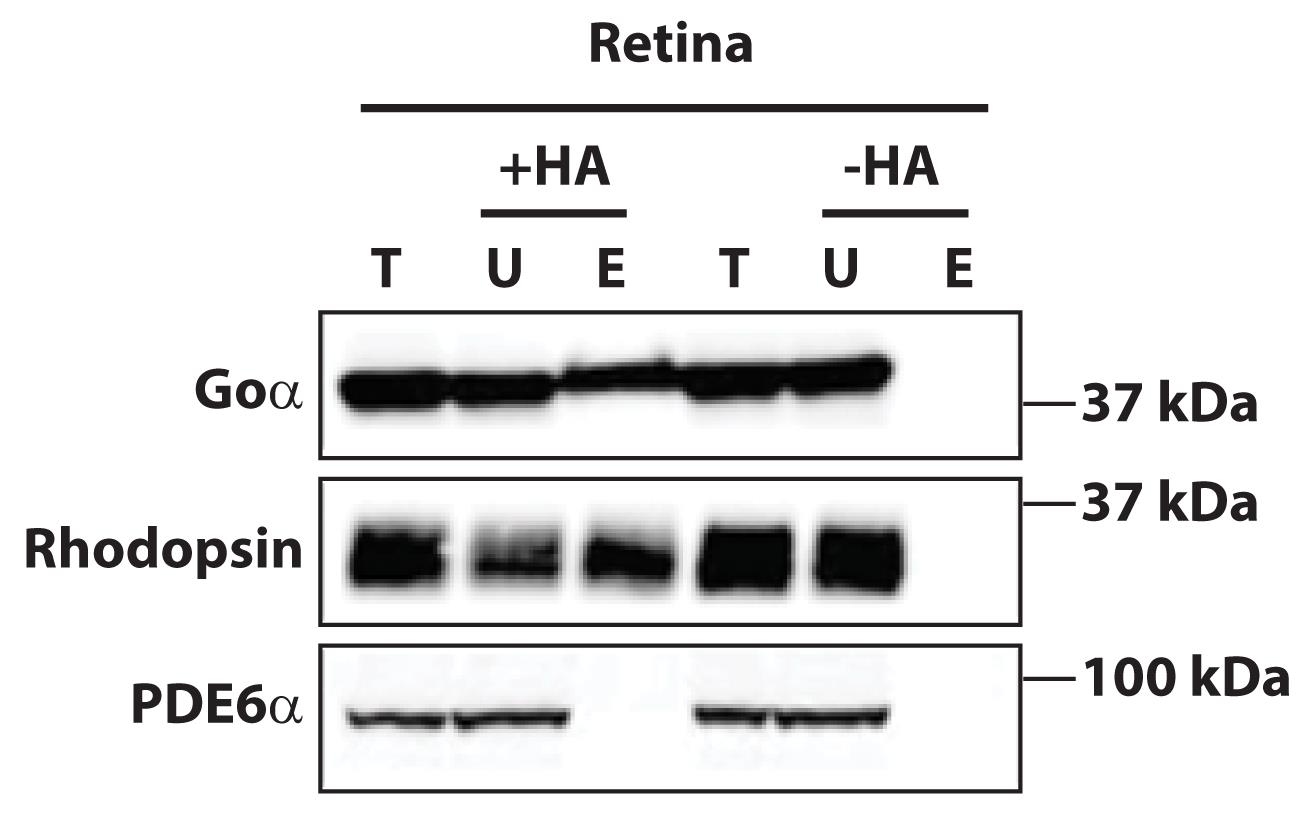
Figure 1. Acyl resin–assisted capture (Acyl-RAC) validation by immunoblotting of known palmitoylated proteins. Purification and detection of known palmitoylated proteins Goα (top panel) and rhodopsin (middle panel) in the wild-type mice retina using thiopropyl Sepharose 6B beads. Phosphodiesterase-6 (PDE6α), a prenylated protein, is not palmitoylated and serves as a control (bottom panel). HA = Hydroxylamine, T = Total, U = Unbound, E = Eluted. Elution in the presence of HA (+HA) represents the isolation of palmitoylated proteins fraction. Elution in the absence of HA (-HA) is used as an internal control to validate the purification scheme.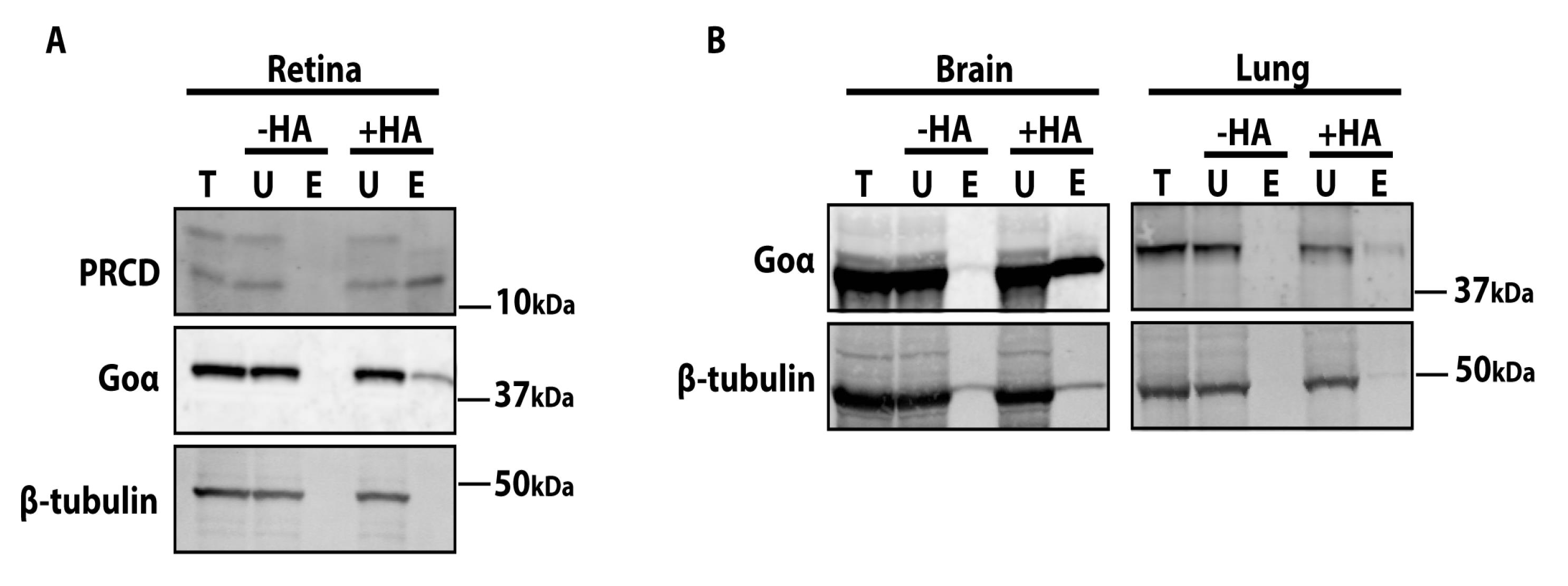
Figure 2. Western blots demonstrating the purification of palmitoylated proteins using agarose S3 beads. A. Palmitoylated proteins isolated from retinal extracts using agarose S3 high-capacity beads were separated by 4%–20% SDS-PAGE and immunoblotted with known palmitoylated proteins, namely progressive rod-cone degeneration (PRCD, top panel) and Goα (middle panel). β-tubulin (bottom panel) is not palmitoylated and serves as a control. B. Western blots showing efficient isolation of palmitoylated proteins from other tissues (brain and lung) using the present Acyl-RAC protocol. Blots are probed with Goα (positive control, top panel) and β-tubulin (negative control, bottom panel).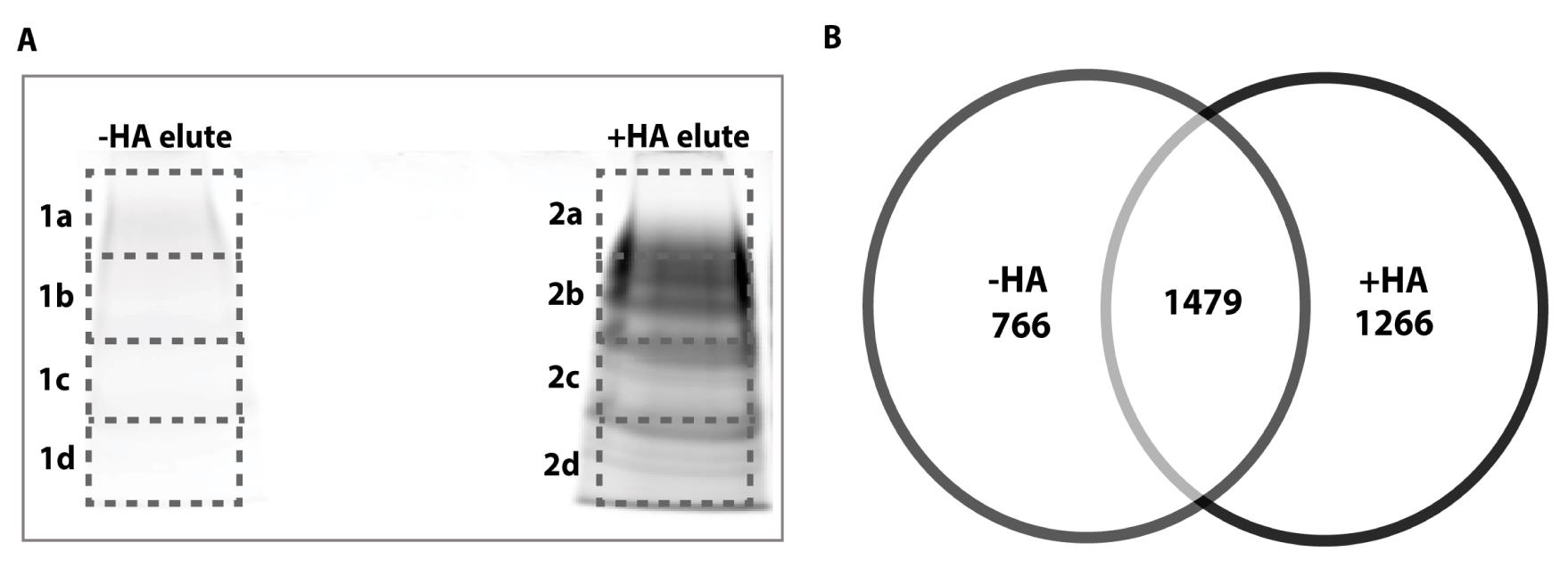
Figure 3. Retinal palmitoyl proteins identified through LC-MS/MS. A. SDS-PAGE followed by Coomassie staining: elutes from -HA (left lane, control) and +HA (right lane) beads were separated on a 10% polyacrylamide gel and stained with Coomassie blue for 1 h at RT, followed by overnight destaining with autoclaved Milli-Q water to visualize the protein bands. The protein bands were cut (as indicated by dotted lines) with a clean blade to perform in-gel digestion, and samples were sent to Harvard Mass spec core for LC-MS/MS to identify retinal palmitoyl proteins. B. Venn diagram showing the total number of unique proteins detected in -HA and +HA fractions. The overlap indicates non-specific proteins detected in both fractions.
Data analysis
Using Microsoft Excel, we identified potential palmitoylated proteins exclusively present in the +HA fraction. Proteins detected in both +HA and -HA fractions are considered non-specific proteins and were excluded from the analyses. To validate the protocol, we show the list of previously known palmitoylated proteins (Table 1) and uncharacterized palmitoylated proteins (Table 2) that are captured by the assay, based on comparisons with published literature. CSS Palm 4.0 – Palmitoylation Sites Prediction software was used to predict the potential palmitoylation sites, with high/medium confidence thresholds, for all the proteins listed in the tables (Ren et al., 2008).
Table 1. Known palmitoylated proteins captured by Acyl-RAC. A list of retinal palmitoylated proteins detected in the +HA fraction by LC-MS/MS that were compared with published literature to confirm the palmitoylation status
| Accession # | Description | Symbol | Mol. Wt (kDa) | CSS-Palm Predicted palmitoylation residue(s) position | Coverage | Peptides | Function |
|---|---|---|---|---|---|---|---|
| 254039605 | Progressive rod-cone degeneration protein homolog precursor [Mus musculus] | PRCD | 6 | 2 (Medium) | 35.8 | 1 | Photoreceptor disc formation |
| 21717805 | Rhodopsin [Mus musculus] | Rho | 39 | 322, 323 (High) | 31.9 | 13 | Phototransduction |
| 71892412 | Retinol dehydrogenase 8 [Mus musculus] | RDH8 | 34.67 | None | 21.5 | 6 | Converts all-trans-retinal to all-trans-retinol |
| 568921542 | Ankyrin-2 isoform X8 [Mus musculus] | ANK-2/ANK-B | 441 | 1451 (High) | 22.6 | 60 | Lens architecture and biomechanics |
| 9055356 | Syntaxin-8 [Mus musculus] | STX8 | 26.9 | 214 (Medium) | 22.9 | 5 | Vesicle trafficking protein |
| 12025532 | V-type proton ATPase 116 kDa subunit a isoform 1 isoform 1 [Mus musculus] | V-ATPase | 96.23 | 24, 310 (High) | 31.7 | 24 | ATP hydrolysis-driven proton pumps |
| 569019533 | Flotillin-1 isoform X1 [Mus musculus] | Flot-1 | 47.84 | 5, 17 (High) Cys34; experimentally proved | 64.5 | 24 | Cell proliferation, T-cell activation, and phagocytosis |
| 31543797 | Synaptotagmin-2 [Mus musculus] | SYT2 | 47.21 | 82, 83, 85, 87, 90, 91, 92 (High) | 42.9 | 17 | Trigger Ca2+-dependent neurotransmitter release |
| 239985643 | Paralemmin-1 isoform 2 [Mus musculus] | PALM | 36.69 | 335, 336 (High) | 77 | 27 | Regulator of filopodia induction and spine maturation |
| 568916068 | Synaptosomal-associated protein 23 isoform X2 [Mus musculus] | SNAP23 | 24.53 | 79, 80, 83, 85 (High) | 69.2 | 13 | Vesicular membrane fusion machinery |
| 31982233 | Lyric [Mus musculus] | AEG-1 | 63.8 | None | 42.8 | 18 | NF-kappaB binding activity; double-stranded RNA binding activity; and transcription coactivator activity |
| 6677873 | Stathmin-3 [Mus musculus] | STMN3 | 20.94 | 22, 24 (High) | 25 | 5 | Microtubule destabilizing activity, regulation of GTPase activity |
| 194328695 | Phospholipid scramblase 1 [Mus musculus] | PLSCR1 | 35.89 | 157, 162, 163, 190, 193, 194, 197, 198, 248, 249 (High) | 18.6 | 6 | Transcriptional activator activity, EGFR binding, DNA binding, apoptosis, immune response |
| 194363764 | GTPase HRas isoform 1 [Mus musculus] | HRAS | 21.28 | 181, 184 (High) | 62.4 | 11 | GTPase activity, cell cycle, signaling |
| 19526820 | Protein XRP2 isoform a [Mus musculus] | XRP2 | 39.35 | 3, 4, 127 (High) | 23.6 | 9 | GAP activity, protein transport |
| 11596855 | Transferrin receptor protein 1 [Mus musculus] | TfR1 | 85.67 | 98 (medium) | 16.9 | 10 | Cellular iron uptake |
| 11612509 | Ras-related protein Ral-B precursor [Mus musculus] | RALB | 23.33 | 203, 204 (high) | 23.3 | 5 | Cell cycle, apoptosis |
| 304307779 | Phosphoprotein associated with glycosphingolipid-enriched microdomains 1 [Mus musculus] | PAG1 | 46.52 | 39, 42 (High) | 24 | 7 | SH2 domain binding, immune response, signal transduction |
| 30520123 | Phospholipid scramblase 4 [Mus musculus] | PLSCR4 | 36.55 | 193, 194, 195, 197, 198, 200 (High) | 11 | 3 | Ion binding, migration of bilayer |
| 6680878 | Lysosome membrane protein 2 [Mus musculus] | LIMP2 | 54 | 4, 5 (High) | 22.6 | 8 | Lysosomal receptor for GBA-targeting, cell adhesion, protein targeting to lysosome |
Table 2. Potentially palmitoylated retinal proteins captured by Acyl-RAC.
A list of uncharacterized retinal palmitoylated proteins detected in the +HA fraction by LC-MS/MS.
| Accession # | Description | Symbol | Mol. Wt (kDa) | CSS-Palm Predicted palmitoylation residue(s) position | Coverage | Peptides | Function |
|---|---|---|---|---|---|---|---|
| 306482605 | Cyclic nucleotide-gated cation channel beta-1 isoform 1 [Mus musculus] | CNGAb1 | 148.62 | 707, 708 (High) | 32.8 | 34 | Phototransduction |
| 10946800 | Syntaxin-6 [Mus musculus] | STX6 | 28.97 | 236 (Medium) | 45.9 | 7 | Trans-Golgi network vesicle trafficking |
| 755565489 | Plasma membrane calcium-transporting ATPase 3 isoform X1 [Mus musculus] | ATPase3 | 137.5 | None | 28.2 | 32 | Ca2+ extrusion |
| 254675320 | Prominin-1 isoform s8 precursor [Mus musculus] | Prom1 | 94.41 | 14, 129, 134, 136, 137, 154, 308 (High) | 24.5 | 19 | Required for retinal development and photoreceptor disc morphogenesis |
| 113680348 | Fascin [Mus musculus] | FSCN2 | 54.47 | 19 (High) | 24.7 | 9 | Actin binding, cytoskeletal |
| 115749432 | Grifin [Mus musculus] | GRIFN | 15.96 | 9 (Medium) | 16 | 2 | Lens-specific protein that binds to alpha-crystallin |
| 568889996 | Ubiquitin-conjugating enzyme E2 L3 [Mus musculus] | UBE2L3 | 17.85 | 17 (high) | 21.4 | 2 | Critical role in the UPS |
| 568986868 | Kinectin isoform X1 [Mus musculus] | KTN1 | 152.44 | None | 10.6 | 8 | Facilitates ER transport along microtubules in association with kinesin to support focal adhesion growth |
| 21313162 | Ras-related protein Rab-1B [Mus musculus] | Rab1B | 22.17 | 200 (High) | 61.2 | 10 | Intracellular membrane trafficking, protein transport, autophagy, vesicle fusion |
| 568928433 | Sodium- and chloride-dependent glycine transporter 1 isoform X2 [Mus musculus] | SLC6A9 | 72.312 | 490 (High) | 17.5 | 9 | Amino acid transporter, neurotransmitter transport |
Recipes
Lysis buffer base
25 mM HEPES (1.25 mL of 1 M HEPES, pH 7.5)
25 mM NaCl (250 µL of 5 M NaCl)
1 mM EDTA (100 µL of 500 mM EDTA)
Up to 50 mL with Milli-Q water
Store at RT
Lysis buffer – working solution
10 mL of lysis buffer base
1 Pierce protease inhibitor mini tablet, EDTA-free
10 µL of phosphatase inhibitor
10 µL of HDSF (depalmitoylation inhibitor)
Store at -20 °C
Binding buffer
100 mM HEPES (5 mL of 1 M HEPES, pH 7.5)
1 mM EDTA (100 µL of 500 mM EDTA)
1% SDS (5 mL of 10% SDS solution)
Up to 50 mL with reverse osmosis (RO) water
Store at RT
Blocking buffer
100 mM HEPES (5 mL of 1 M HEPES, pH 7.5)
1 mM EDTA (100 µL of 500 mM EDTA)
2.5% SDS (12.5 mL of 10% SDS solution)
Up to 50 mL with RO water
Store at RT
5× Laemmli sample buffer
3.0 g of SDS
15 mL of 0.5 M Tris-HCl, pH 6.8
15 mL of 100% glycerol
0.02% bromophenol blue
Aliquot 950 µL per tube and store at -20 °C
Before use, add 50 µL of β-ME and 10 mM DTT (10 µL of 1 M DTT) to the tube and vortex to mix thoroughly.
1× Laemmli sample buffer
Dilute 5× Laemmli sample buffer (with β-ME and DTT) with autoclaved distilled/Milli-Q water. Vortex to mix thoroughly. Store at -20 °C.
1 M dithiothreitol (DTT)
154.2 mg of DTT
1 mL of autoclaved Milli-Q water
Aliquot and store at -20 °C
100% TCA stock preparation (w/v)
Dissolve 27.5 g of TCA in 20 mL of Milli-Q water.
After dissolving, make up to 50 mL with Milli-Q water
Store at 4 °C
1× SDS-PAGE running buffer
500 mL of 10× Tris-glycine SDS running buffer
4500 mL of RO water
Mix thoroughly and store at RT
70% acetone
70 mL of 100% acetone + 30 mL of autoclaved Milli-Q water
Store at RT
2 M hydroxylamine (freshly prepare for every assay)
69.49 mg of hydroxylamine-HCl
Dissolve with 300 µL of autoclaved Milli-Q water
Adjust to pH 7.5 with NaOH
Make up to 500 µL with autoclaved Milli-Q water
2 M sodium chloride
200 µL of 5 M NaCl + 300 µL of autoclaved Milli-Q water
Store at RT
5 M sodium hydroxide
Dissolve 10 g of NaOH pellets in 40 mL of autoclaved Milli-Q water and let it cool
Bring the final volume to 50 mL
Store at RT in the corrosive cabinet
10% Triton-X 100
Dissolve 5 mL of Triton X-100 in 45 mL of autoclaved Milli-Q water
Mix thoroughly and store at 4 °C
Acknowledgments
This research was supported by the National Institutes of Health, grant number RO1EY028959 (SK), and West Virginia University Bridge funding (SK). Also, we thank Dr. Ramamurthy for his valuable suggestions and support in developing this acyl-RAC protocol.
Competing interests
The authors declare no competing interests.
Ethics
The experimental procedures involving animals were performed in agreement with National Institutes of Health (NIH) guidelines. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at West Virginia University.
References
Baker, S. A., Haeri, M., Yoo, P., Gospe, S. M., Skiba, N. P., Knox, B. E. and Arshavsky, V. Y. (2008). The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J Cell Biol 183(3): 485-498.
- Burns, M. E. and Arshavsky, V. Y. (2005). Beyond Counting Photons: Trials and Trends in Vertebrate Visual Transduction. Neuron 48(3): 387-401.
- Edmonds, M. J., Geary, B., Doherty, M. K. and Morgan, A. (2017). Analysis of the brain palmitoyl-proteome using both acyl-biotin exchange and acyl-resin-assisted capture methods. Sci Rep 7(1): 1-13.
- Forrester, M. T., Hess, D. T., Thompson, J. W., Hultman, R., Moseley, M. A., Stamler, J. S. and Casey, P. J. (2011). Site-specific analysis of protein S-acylation by resin-assisted capture.J Lipid Res 52(2): 393.
- Kolandaivelu, S., Huang, J., Hurley, J. B. and Ramamurthy, V. (2009). AIPL1, a protein associated with childhood blindness, interacts with α-subunit of rod phosphodiesterase (PDE6)and is essential for its proper assembly. J Biol Chem 284(45): 30853-30861.
- Maeda, A., Okano, K., Park, P. S. H., Lem, J., Crouch, R. K., Maeda, T. and Palczewski, K. (2010). Palmitoylation stabilizes unliganded rod opsin. Proc Natl Acad Sci U S A 107(18): 8428-8433.
- Murphy, J. and Kolandaivelu, S. (2016). Palmitoylation of Progressive Rod-Cone Degeneration (PRCD) Regulates Protein Stability and Localization. J Biol Chem 291(44): 23036-23046.
- Myers, B., Sechrest, E. R., Hamner, G., Motipally, S. I., Murphy, J. and Kolandaivelu, S. (2022). R17C Mutation in Photoreceptor Disc-Specific Protein, PRCD, Results in Additional Lipidation Altering Protein Stability and Subcellular Localization. Int J Mol Scis 23(18): 10802.
- Ren, J., Wen, L., Gao, X., Jin, C., Xue, Y. and Yao, X. (2008). CSS-Palm 2.0: an updated software for palmitoylation sites prediction. Protein Eng Des Sel 21(11): 639-644.
- Yang, Z. and Wensels, T. G. (1992). N-Myristoylation of the Rod Outer Segment G Protein, Transducin, in Cultured Retinas. J Bio Chem 267(32): 23197-23201.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Motipally, S. I., Myers, B., Sechrest, E. R., Sokolov, D., Murphy, J. and Kolandaivelu, S. (2023). A Modified Acyl-RAC Method of Isolating Retinal Palmitoyl Proteome for Subsequent Detection through LC-MS/MS. Bio-protocol 13(8): e4654. DOI: 10.21769/BioProtoc.4654.
Category
Biochemistry > Protein > Posttranslational modification
Neuroscience > Sensory and motor systems > Retina
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









