- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assessment of Chemosensory Response to Volatile Compounds in Healthy, Aged, and Neurodegenerative Caenorhabditis elegans Models
Published: Vol 13, Iss 9, May 5, 2023 DOI: 10.21769/BioProtoc.4650 Views: 1522
Reviewed by: Juan Facundo Rodriguez AyalaDurai SellegounderRajesh RanjanAyush Ranawade

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Analysis of Mitochondrial Structure in the Body Wall Muscle of Caenorhabditis elegans
Shaarika Sarasija and Kenneth R. Norman
Apr 5, 2018 9432 Views

Imaging and Fluorescence Quantification in Caenorhabditis elegans with Flow Vermimetry and Automated Microscopy
Elissa Tjahjono [...] Natalia V. Kirienko
May 20, 2021 4891 Views

TUNEL Labeling to Detect Double-stranded DNA Breaks in Caenorhabditis elegans Gonads
Peter A. Kropp [...] Andy Golden
Mar 20, 2022 2620 Views
Abstract
A basic function of the nervous system is to confer the ability to detect external stimuli and generate appropriate behavioral and physiological responses. These can be modulated when parallel streams of information are provided to the nervous system and neural activity is appropriately altered. The nematode Caenorhabditis elegans utilizes a simple and well characterized neural circuit to mediate avoidance or attraction responses to stimuli, such as the volatile odorant octanol or diacetyl (DA), respectively. Aging and neurodegeneration constitute two important factors altering the ability to detect external signals and, therefore, changing behavior. Here, we present a modified protocol to assess avoidance or attraction responses to diverse stimuli in healthy individuals and Caenorhabditis elegans models associated with neurodegenerative diseases.
Keywords: Caenorhabditis elegansBackground
A basic function of the nervous system is to confer the ability to detect external stimuli and generate appropriate behavioral and physiological responses (Baidya et al., 2014). Caenorhabditis elegans has only fourteen types of chemosensory neurons but responds to dozens of chemicals, because each chemosensory neuron detects a wide variety of volatile (olfactory) and water-soluble (gustatory) cues associated with food, danger signals, or other animals (Troemel et al., 1995; Dosanjh et al., 2010). Much of its nervous system and more than 5% of its genes are devoted to the recognition of environmental chemicals. Chemosensory cues can elicit chemotaxis, rapid avoidance, changes in overall motility, and entry into or exit from the alternative dauer developmental stage. These behaviors are regulated by eleven pairs of chemosensory neurons. Each amphid sensory neuron expresses a specific set of candidate receptor genes and detects a characteristic set of attractants, repellents, or pheromones (Bargmann, 2006). Olfaction is a versatile and sensitive mechanism for detecting volatile odorants. Many of these responses are mediated (at least in part) by a pair of ciliated sensory neurons named ASH, which have sensory openings at the anterior amphid pore at the nose of the animal and are thought to be analogous to polymodal nociceptive neurons in vertebrates; other sensory neurons (e.g., ADL, AWB, ASK) are thought to play minor and auxiliary roles in avoidance responses (Figure 1). The avoidance response to 30% octanol via ASH neurons is enhanced by serotonin; furthermore, altering serotonin levels results in ADL and AWB neurons being recruited to detect 100% octanol redundantly with ASH neurons (Chao et al., 2004). In the nematode, the presence of this compound has a strong modulatory influence on many behaviors. For instance, the olfaction of octanol or diacetyl (DA) stimulates C. elegans mechanosensory neurons to release dopamine, which affects many behaviors including locomotion rate (Sawin et al., 2000), foraging, and response to soluble repellants (Ezcurra et al., 2011). In this protocol, we describe how to evaluate the time response against attractants and repellents in the nematode C. elegans to study the neuronal system and behavioral response of worms, and how this can be translated to neuronal diseases in worms fed on different bacteria. For example, as worms age, they have more difficulty sensing octanol and their avoidance response slows, the same being observed with the DA (Figure 1). This protocol has the advantage of not requiring a centrifugation step, which could severely damage aged and sick worms. As far as we know, this protocol is the first one to be applied to assess neurological damage in C. elegans.
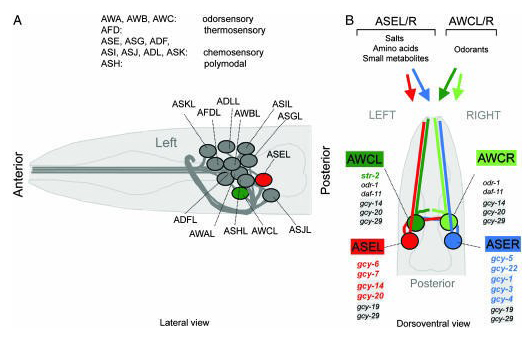
Figure 1. Introduction to C. elegans sensory anatomy. (A) A prominent and well-characterized subset of C. elegans sensory neurons, the amphid sensory neurons. As with most other neuron classes, amphid sensory neuron classes consist of one pair of bilaterally symmetric cells (see also B). Each of the 12 pairs of amphid sensory neurons extends a dendrite to the tip of the nose and an axon into the nerve ring, a nerve bundle where synaptic connections are made (White et al., 1986). Delineated functions of amphid sensory neurons are indicated (Bargmann and Mori 1997). (B) Amphid sensory neuron classes consist of two bilaterally symmetric pairs of neurons, two of which, AWCL/R and ASEL/R, are functionally lateralized. While some other amphid sensory neurons appear to contribute to gustation, ASE is the main gustatory neuron class in C. elegans, mediating responses to salts, amino acids, and small metabolites (Bargmann et al., 1993). ASE mediates not only attractive but also repulsive responses to specific chemicals (Sambongi et al., 1999). The salts sodium, chloride, and potassium are sensed in a left/right asymmetric manner, with ASEL sensing sodium but not chloride and potassium, and ASER sensing chloride and potassium (Pierce-Shimomura et al., 2001). It is not yet known whether other ASE-sensed chemicals may also activate ASEL and ASER differentially. ASE-expressed GCY genes are shown and those that are asymmetric are colored. The AWCL/R neurons can discriminate benzaldehyde and butanone on the basis of the left/right asymmetric expression of str-2 (colored), a G-protein coupled receptor, which is stochastically expressed in either AWCL or AWCR (Troemel et al., 1999; Wes and Bargmann, 2001). Newly identified GCY genes in AWC are shaded. Figure taken from Ortiz et al. (2006).
Materials and Reagents
Glass media bottles 200 mL (Fisher Scientific, catalog number: FB800250).
Petri dishes 60 × 15 mm 500/cs (Fisher Scientific, catalog number: FB0875713A)
Pipette tips 2–200 µL Eppendorf® epT.I.P.S. (Eppendorf, catalog number: 022492039)
Pipette tips 50–1,000 µL Eppendorf® epT.I.P.S. (Eppendorf, catalog number: 022492055)
Eppendorf® Safe-Lock 1.5 mL microcentrifuge tubes (Eppendorf, catalog number: 022363204)
Corning® 15 mL centrifuge tubes (Corning, catalog number: 430791)
C. elegans strains were obtained from Caenorhabditis Genetics Center: N2 Bristol (WormBase ID: WBStrain00000001; dvIs15), CL2122 (WBStrain00005101), and CL2355 [WBStrain00005106; smg-1(cc546); pCL45 [Psnb-1::human Amyloid beta 1-42::3' UTR (long); Pmtl-2::GFP]]
Escherichia coli strain OP50 (University of Minnesota, C. elegans Genetics Center, MN)
Bacillus subtilis strain NCIB3610 (Bacillus Genetic Stock Center, catalog numbers: 3A1 and 1A96)
Magnesium sulfate heptahydrate (MgSO4·7H2O) (Sigma-Aldrich, catalog number: M1880)
Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655)
Potassium phosphate dibasic (K2HPO4) (Sigma-Aldrich, catalog number: P2222)
Sodium phosphate dibasic heptahydrate (Na2HPO4·7H2O) (Sigma-Aldrich, catalog number: 7782-85-6)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653)
NaClO hypochlorite (Sigma-Aldrich, catalog number: 13440)
Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: S8045)
Bacto peptone (BD, BactoTM, catalog number: 211677)
Agar (Sigma-Aldrich, catalog number: A1296)
Cholesterol (Sigma-Aldrich, catalog number: C8667)
100% ethanol (Sigma-Aldrich, catalog number: E7023)
Calcium chloride dihydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C3881)
Luria broth (LB) (Sigma-Aldrich, catalog number: L3522)
Octanol (1-octanol) (Sigma-Aldrich, catalog number: 472328)
Diacetyl (DA) (butane-2,3-dione) (Sigma-Aldrich, catalog number: B85307)
M9 buffer (see Recipes)
Nematode growth medium (NGM) agar (see Recipes)
Potassium phosphate buffer (see Recipes)
1 M MgSO4·7H2O (see Recipes)
Cholesterol in absolute ethanol (5 mg/mL) (see Recipes)
1 M CaCl2 (see Recipes)
Potassium phosphate buffer (1 M KPO4, pH 6.0) (see Recipes)
LB media (see Recipes)
Bleaching solution (see Recipes)
1 N NaOH (see Recipes)
0.5% DA (see Recipes)
S-basal buffer (see Recipes)
Equipment
Shaker (Axyos Technologies, catalog number: S04036.tu)
Dissecting microscope (Carl Zeiss, catalog number: 3944010970)
Commercially available paintbrush number: 10/0; 0.2 × 0.2 × 6.5 inches (Princeton art & Brush, catalog number: B0043GCYSI).
Autoclave (Tuttnauer USA, model: 6690)
Worm pick. Worm picks can either be purchased (Genesee Scientific, catalog number: 59-AWP) or made in the lab as described in Wollenberg et al. (2013).
Centrifuge (Eppendorf, model: 5430)
Tabletop centrifuge (Eppendorf, model: 5424)
Bunsen burner (Humbolt, catalog number: H-5870)
Chronometer (Hinotek, catalog number: PS-360A)
Software
Microsoft Excel (Microsoft Corporation, Redmond, USA)
GraphPad Prism 8 (GraphPad Software, Inc.)
Procedure
C. elegans maintenance
Inoculate an E. coli OP50 colony into 50 mL of Luria-Bertani (LB) broth in a 200 mL flask and incubate with gentle agitation at 37 °C overnight.
Seed approximately 200 μL of E. coli OP50 overnight culture (1 × 108 CFU/mL) to a 60 mm NGM agar plate. Incubate plates at 37 °C overnight before use.
Transfer 50–100 mixed-stage larval worms (N2, CL2122, and CL2355 strains) to a new E. coli OP50-seeded NGM agar plate using a heat-sterilized platinum wire pick. Keep worms at 20 °C.
Repeat step A3 every 2–3 days for maintenance.
C. elegans synchronization by bleaching
Allow worms to grow until adult stage.
Recover gravid adults in 15 mL tubes by washing plates with M9 buffer (see Recipe 1).
Pellet worms by centrifuging for 2 min at 400 × g (~1,500 rpm on a standard table centrifuge) at room-temperature and discard supernatant.
Perform 1–3 washes until the buffer appears clear of bacteria (lack of turbidity).
Add 5 mL of bleaching solution (see Recipe 8) into the tube and shake vigorously at room temperature for up to 5 min. Bleaching longer than 5 min will kill the eggs.
Dilute the reaction by adding 10 mL of S-basal buffer to the 15 mL centrifuge tube.
Quickly centrifuge (since treatment may still be active) for 1 min at 400 × g and discard supernatant.
Wash pellet three more times by filling the tube with S-basal buffer. Check using a dissecting microscope.
Place the eggs to unseeded NGM plates and incubate for 18–24 h at 20 °C. Proper aeration should be provided to obtain all animals in stage L1.
Wash each NGM plate with 1 mL of S-basal buffer, transfer the L1 population to NGM agar plates seeded with the corresponding bacterial food, and incubate until they reach the young-adult stage (1-day-old L4), approximately 48 h later. Most of the C. elegans strains are maintained at 20 °C on NGM media seeded with E. coli OP50 or B. subtilis NCIB3610.
Behavior assay
Feed C. elegans N2, CL2122, and CL2355 strains on E. coli OP50 or B. subtilis NCIB3610 bacterial cells from the L1-larval stage to adulthood at 20 °C.
Repulsion and attraction behavioral assays are performed using octanol or DA as repellent or attractant agents, respectively.
Wash E. coli OP50 or B. subtilis NCIB3610 strain-fed adult worms of different ages three times with M9 buffer to remove any residual bacteria and place them in NGM plates without food.
For the repellent assay, 1 h after food starvation, place a paintbrush hair previously dipped in 100% octanol solution in front of a moving animal.
Score the escape response time to octanol as the time (s) from exposure to the repellent to the initiation of a backward or escape movement.
To measure the reaction time against octanol, use a timer that is stopped when the worm begins its regression, making a movement backwards (Figures 3 and 4).
For the attractant assay, 1 h after food starvation, place a paintbrush hair previously dipped in 0.5% DA in ethanol 1.5 cm in front of a moving animal.
Note: Mark the distance of 1.5 cm (with two dots or lines, one for the place where the brush is and another where the worms will be) outside the Petri dish in the attraction test so that the test is reproducible (Figure 2).
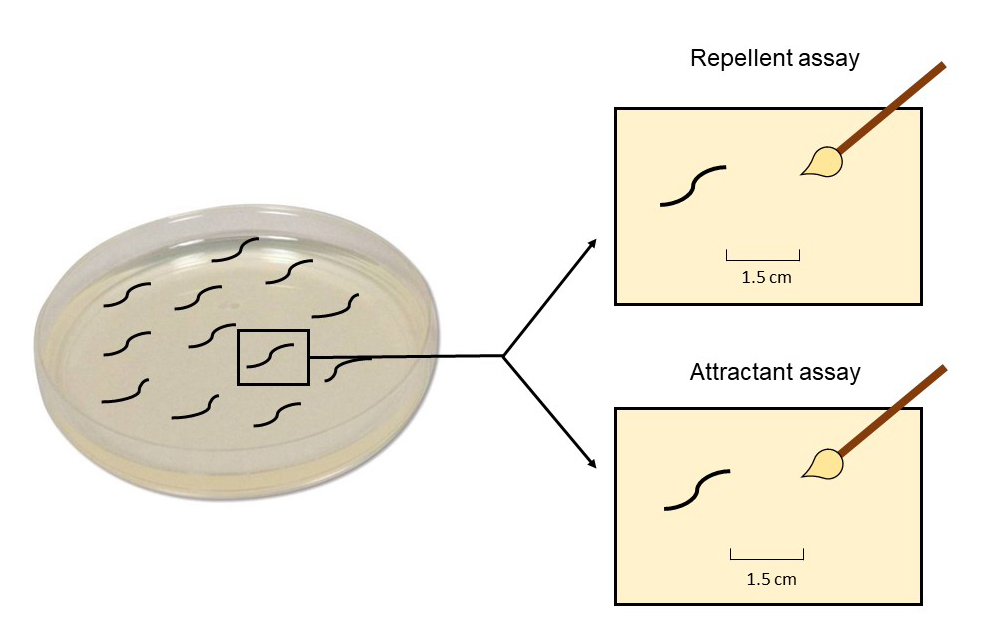
Figure 2. Scheme of the response time assay of C. elegans against the repellent octanol or the attractive diacetyl (DA). In the repellent assay, put the paintbrush dipped in octanol in front of the worm’s head and start the chronometer. Stop it when the worm begins its regression. Do the same for the attraction assay, but in this case put the paintbrush dipped in DA 1.5 cm in front of the worm and stop the chronometer when the worm initiates its forward movement in the direction of DA. The movement of the worm can be visualized in the following link: https://www.youtube.com/watch?v=klOJb0DDGGU&ab_channel=NeuroAIUWLaboratory. As can be seen in the video, ALM+AVM neural stimulus is capable of generating the avoidance behavior; the stimulus disturbs forward locomotion and initiates backward locomotion. Inspection of neural activity of motor neurons (DB neurons are anterior -> posterior ordered) indicates that the stimulus induces a change in the directionality of neural activity traveling wave, from anterior -> posterior to posterior -> anterior. The transition is marked through high constant activity in the anterior motor neurons.Score the DA response time as the time (s) from the DA presentation to the initiation of a forward movement in the direction of DA (Figure 3 and 4).
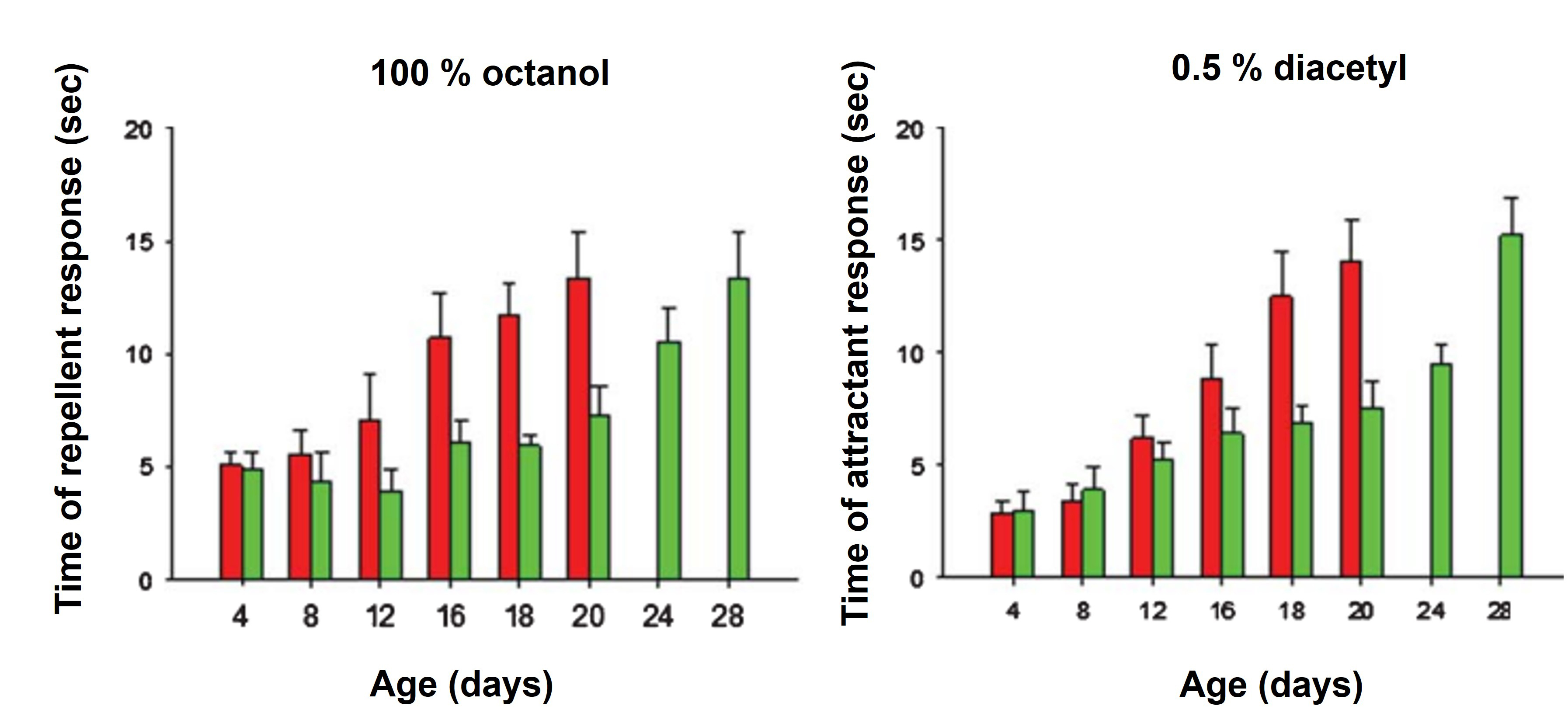
Figure 3. B. subtilis-mediated cognitive improvement during C. elegans aging. Average response times (in seconds, y-axis) of OP50- and NCIB3610-colonized N2 worms (red and green columns, respectively) of different ages (in days, x-axis) to repellent (octanol, left) and attractant [diacetyl (DA), right] exposition. Results represent mean ± standard deviation of three independent experiments performed in duplicate. A typical result from one of the three independent experiments performed in duplicate is presented (mean ± S.E.M). From Cogliati et al. (2020).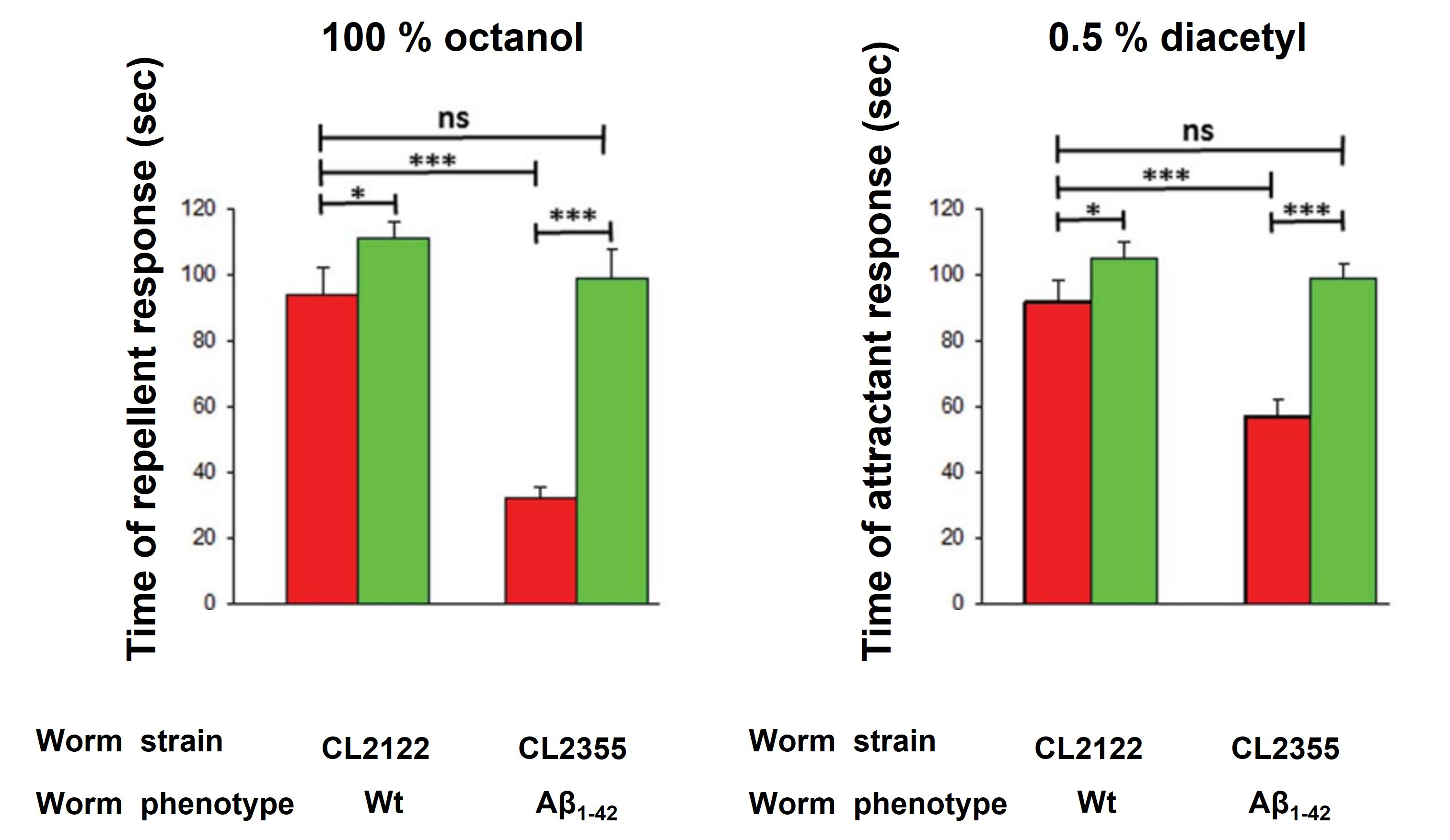
Figure 4. B. subtilis improved the behavioral response of C. elegans expressing pan-neuronal Aβ aggregates. Time of response of OP50- and NCIB3610-colonized (red and green columns, respectively) CL2122 (control or CL2122 wt, left) and CL2355 [CL2355 Alzheimer’s disease (AD) model expressing the pan-neuronal Aβ1-42, right] worms to 100% octanol (repellent), and CL2122 (control or CL2122 wt, left) and CL2355 (AD model expressing the pan-neuronal Aβ1-42, right) worms to 0.5% diacetyl (DA, attractant). A typical result out of three independent experiments (performed in duplicate) is presented (mean ± standard deviation). Asterisks indicate statistical significance (*p < 0.1; ***p < 0.001; ns, no significant difference, p > 0.5).
Data analysis and interpretation
All assays were performed at least three times in duplicate. In the repellent assay, put the paintbrush dipped in octanol in front of the worm’s head and start the chronometer. Stop it when the worms begin their regression. This represents the time of repellent response in seconds. Do the same for the attraction assay, but in this case put the paintbrush dipped in DA 1.5 cm in front of the worm and stop the chronometer when the worms initiate its forward movement in the direction of DA. This represents the time of attraction response in seconds. Calculate the time of attraction response and the time of repellent response and plot the data in Microsoft Excel. Use GraphPad Prism 8 for statistical analysis of data. Combine data from three independent experiments and calculate the mean and standard deviation. Apply a Student’s t-test with a significance cut-off level of P < 0.05 for comparisons between two groups for attraction and repellent time.
Recipes
M9 buffer
3 g of KH2PO4
6 g of Na2HPO4·7H2O
5 g of NaCl
Water up to 1 L. Autoclave, then add 1 mL of 1 M MgSO4.
Nematode growth medium (NGM) agar for 1 L medium
3.0 g of NaCl
20 g of agar
2.5 g of Bacto peptone
Water up to 972 mL. Autoclave to sterilize the agar, cool to 55 °C, then add:
1 mL of 1 M MgSO4·7H2O (see Recipe 3)
1 mL of 1 M CaCl2 (see Recipe 5)
1 mL of 5 mg/mL cholesterol in absolute ethanol (see Recipe 4)
25 mL of 1 M potassium phosphate buffer pH 6.0 (see Recipe 6)
1 M MgSO4·7H2O
Dissolve 6 g of MgSO4 heptahydrate in 50 mL of dH2O
Autoclave (121 °C for 20 min)
Store at room temperature.
Cholesterol in absolute ethanol (5 mg/mL)
Dissolve 0.25 g of cholesterol in 50 mL of 100% ethanol
Do not autoclave.
1 M CaCl2
Dissolve 5.55 g of CaCl2 dihydrate in 50 mL of dH2O
Autoclave (121 °C for 20 min)
Store at room temperature.
Potassium phosphate buffer (1 M KPO4, pH 6.0)
108.3 g of KH2PO4
35.6 g of K2HPO4
Water up to 1 L
Autoclave (121 °C for 20 min)
Store at room temperature.
LB media
Dissolve 20 g of LB broth in 1 L of ddH2O
Autoclave (121 °C for 20 min).
Bleaching solution
1 mL of 3% NaClO hypochlorite
2.5 mL of 1 N NaOH (see Recipe 9)
0.5 mL of dH2O
1 N NaOH
Dissolve 2 g of NaOH in 50 mL of dH2O
0.5% DA
Dissolve diacetyl in 100% ethanol. Diacetyl should be prepared immediately prior to any experiments by dilution in ethanol.
S-basal buffer
5.85 g of NaCl
1 g of K2HPO4
6 g of KH2PO4
H2O to 1 L. Sterilize by autoclaving (121 °C for 20 min). Add 1 mL of cholesterol (5 mg/mL in ethanol).
Notes
When placing the paintbrush in front of the nematode, take care to not touch the worm nor the surface of the agar plate.
In the repellent assay, sterile water was used instead of octanol as a control and assays were halted at 20 s to account for spontaneous reversals.
Only inversions were scored, as determined by observing the backward movement of the tail. An interruption of forward movement or a head-only retreat was not scored.
In the attractant assay, ethanol was used instead of DA as a control.
Acknowledgments
This work was supported by CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas) and FONCyT (Fondo para la Investigación Científica y Tecnológica) with the aid of the Pew Latin-nAmerican Program in Biological Sciences (Philadelphia, USA), the Fulbright Committeen (Washington, DC, USA) and former Fundación Antorchas (Buenos Aires, Argentina). We modified the media and NGM plate preparation from Stiernagle (2006). We modified C. elegans synchronization from Montserrat Porta-de-la-Riva et al. (2012). The protocol is based on “Dopamine modulation of avoidance behavior in Caenorhabditis elegans requires the NMDA receptor NMR-1” (Baidya et al., 2014).
Competing interests
The authors declare no conflicts of interest.
References
Cogliati, S., Clementi, V., Francisco, M., Crespo, C., Arganaraz, F. and Grau, R. (2020). Bacillus subtilis Delays Neurodegeneration and Behavioral Impairment in the Alzheimer's Disease Model Caenorhabditis elegans. J Alzheimers Dis 73(3): 1035-1052.
- Baidya, M., Genovez, M., Torres, M. and Chao, M. Y. (2014). Dopamine modulation of avoidance behavior in Caenorhabditis elegans requires the NMDA receptor NMR-1. PLoS One 9(8): e102958.
- Bargmann, C. I., Hartwieg, E. and Horvitz, H. R. (1993). Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74(3): 515-527.
- Bargmann, C. I. (2006). Chemosensation in C. elegans. WormBook: 1-29.
- Dosanjh, L. E., Brown, M. K., Rao, G., Link, C. D. and Luo, Y. (2010). Behavioral phenotyping of a transgenic Caenorhabditis elegans expressing neuronal amyloid-beta. J Alzheimers Dis 19(2): 681-690.
- Chao, M. Y., Komatsu, H., Fukuto, H. S., Dionne, H. M. and Hart, A. C. (2004). Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci U S A 101(43): 15512-15517.
- Ezcurra, M., Tanizawa, Y., Swoboda, P. and Schafer, W. R. (2011). Food sensitizes C. elegans avoidance behaviours through acute dopamine signalling. EMBO J 30(6): 1110-1122.
- Ortiz, C. O., Etchberger, J. F., Posy, S. L., Frokjaer-Jensen, C., Lockery, S., Honig, B. and Hobert, O. (2006). Searching for neuronal left/right asymmetry: genomewide analysis of nematode receptor-type guanylyl cyclases. Genetics 173(1): 131-149.
- Pierce-Shimomura, J. T., Faumont, S., Gaston, M. R., Pearson, B. J. and Lockery, S. R. (2001). The homeobox gene lim-6 is required for distinct chemosensory representations in C. elegans. Nature 410(6829): 694-8.
- Porta-de-la-Riva, M., Fontrodona, L., Villanueva, A. and Ceron, J. (2012). Basic Caenorhabditis elegans methods: synchronization and observation. J Vis Exp(64): e4019.
- Sambongi, Y., Takeda, K., Wakabayashi, T., Ueda, I., Wada, Y. and Futai, M. (2000). Caenorhabditis elegans senses protons through amphid chemosensory neurons: proton signals elicit avoidance behavior. Neuroreport 11(10): 2229-2232.
- Sawin, E. R., Ranganathan, R. and Horvitz, H. R. (2000). C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26(3): 619-631.
- Stiernagle, T. (2006). Maintenance of C. elegans. WormBook: 1-11.
- Troemel, E. R., Chou, J. H., Dwyer, N. D., Colbert, H. A. and Bargmann, C. I. (1995). Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83(2): 207-218.
- Wes, P. D. and Bargmann, C. I. (2001). C. elegans odour discrimination requires asymmetric diversity in olfactory neurons. Nature 410(6829): 698-701.
- White, J. G., Southgate, E., Thomson, J. N. and Brenner, S. (1986). The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 314(1165): 1-340.
- Wollenberg, A. C., Visvikis, O., Alves, A.-M. F. and Irazoqui, J. E. (2013). Staphylococcus aureus Killing Assay of Caenorhabditis elegans.Bio-protocol 3(19): e916.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Crespo, C. and Grau, R. (2023). Assessment of Chemosensory Response to Volatile Compounds in Healthy, Aged, and Neurodegenerative Caenorhabditis elegans Models. Bio-protocol 13(9): e4650. DOI: 10.21769/BioProtoc.4650.
Category
Neuroscience > Nervous system disorders > Animal model
Developmental Biology > Cell signaling > Stress response
Cell Biology > Cell signaling > Stress response
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









