- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determining Bone-forming Ability and Frequency of Skeletal Stem Cells by Kidney Capsule Transplantation and Limiting Dilution Assay
Published: Vol 13, Iss 6, Mar 20, 2023 DOI: 10.21769/BioProtoc.4639 Views: 1699
Reviewed by: Olga KopachAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Reprogramming White Fat Cells for Adipose Manipulation Transplantation (AMT) Therapy
Kelly An [...] Nadav Ahituv
Aug 5, 2025 2239 Views
Examining the Roles of m6A Sites in mRNA Using the Luciferase Gene Fused With Mutated RRACH Motifs
Nobuko Katoku-Kikyo and Nobuaki Kikyo
Nov 5, 2025 1934 Views
Vascularization of Human Pancreatic Islets With Adaptive Endothelial Cells for In Vitro Analysis and In Vivo Transplantation
Ge Li [...] Shahin Rafii
Dec 20, 2025 746 Views
Abstract
Adult stem cells not only maintain tissue homeostasis but are also critical for tissue regeneration during injury. Skeletal stem cells are multipotent stem cells that can even generate bones and cartilage upon transplantation to an ectopic site. This tissue generation process requires essential stem cell characteristics including self-renewal, engraftment, proliferation, and differentiation in the microenvironment. Our research team has successfully characterized and isolated skeletal stem cells (SSCs) from the cranial suture called suture stem cells (SuSCs), which are responsible for craniofacial bone development, homeostasis, and injury-induced repair. To assess their stemness features, we have demonstrated the use of kidney capsule transplantation for an in vivo clonal expansion study. The results show bone formation at a single-cell level, thus permitting a faithful assessment of stem cell numbers at the ectopic site. The sensitivity in assessing stem cell presence permits using kidney capsule transplantation to determine stem cell frequency by limiting dilution assay. Here, we described detailed protocols for kidney capsule transplantation and limiting dilution assay. These methods are extremely valuable both for the evaluation of skeletogenic ability and the determination of stem cell frequency.
Background
Conventional methods have shown the skeletogenic abilities of mesenchymal stromal cells (MSCs) isolated from bone marrow and other tissues in a Petri dish. However, in vivo transplantation studies indicate that the majority of MSCs lack engraftment, survival, and differentiation abilities (Caplan and Correa, 2011; Zeitouni et al., 2012). Only a small portion of MSCs are genuine skeletal stem cells (SSCs) (Robey et al., 2014; Sacchetti et al., 2007). In addition, in vitro experiments cannot examine certain features that are essential for stem cell stemness, thus lacking critical characteristics to fit into the rigorous criteria for the modern definition of SSCs. Animal models of ectopic bone formation have clear advantages over orthotopic transplantation because of the environments lacking cytokine interference and interactions with endogenous cell types, e.g., bone-forming cells. Three ectopic locations— subcutaneous, intramuscular, and kidney capsule—have been mainly used for transplantation studies (Scott et al., 2012). Subcutaneous implantation is the simplest method, but pertinent concerns arise because of several caveats related to the paucity of bone formation (Yang et al., 1996). Intramuscular implantation implies close contact with native skeletal muscle progenitors, raising concerns about the cellular origin of the ectopic bone that could be converted from host cells by osteogenic stimulus or traumatic injury (Takaoka et al., 1988; Yu et al., 2008). However, no endogenous cell interferes with the analysis of the transplant, which can be easily identified through kidney capsule transplantation. Although technically more demanding, the environment provides the most nutrient-rich resource for robust bone formation from significantly fewer cell numbers (Maruyama et al., 2021). Using kidney capsule transplantation, we have demonstrated ectopic bone generation at a single-cell level (Maruyama et al., 2016). This method also enables the determination of stem cells’ switch from an osteogenic to a chondrogenic fate for cartilage generation (Maruyama et al., 2016; Maruyama et al., 2022a). Furthermore, because kidney capsule transplantation permits in vivo clonal expansion analysis, it is sensitive enough to determine stem cell frequency by limiting dilution analysis (Maruyama et al., 2016; Maruyama et al., 2021). These analyses utilizing kidney capsule transplantation are powerful tools with clear advantages to advance skeletal stem cell research.
Materials and Reagents
50 mL conical polypropylene centrifuge tube (Fisher Scientific, catalog number: 12-565-271)
1.5 mL microtube (Fisher Scientific, catalog number: 05-408-129)
Insulin syringes with Ultra-FineTM needle (BD Biosciences, catalog number: 08290-3284-38)
Artificial tears ointment (Rugby Laboratories, catalog number: 0536-6550-91)
Cotton swab, puritan 6” sterile (Puritan, catalog number: 25-806 1WC)
Povidone-Iodine cotton swab stick, individual packet 10% strength (Medicine, catalog number: MDS093901)
Alcohol pads (PDI/Professional Disposables, catalog number: B60307)
Nonidet P-40 substitute (Sigma-Aldrich, catalog number: 98379)
Capillary tube (Drummond Scientific, catalog number: 1-000-800)
POLYSYN violet braided coated polyglycolic acid absorbable suture (catalog number: G391NV, TruStitch)
Latex gloves (VWR, catalog number: 56617-180), 5 years shelf life at room temperature
SCID mouse (NOD.CB17-Prkdcscid/NCrCrl, Charles River) older than two months of age
Dulbecco’s phosphate-buffered saline without calcium & magnesium (Gibco, catalog number: 10010-023), two years shelf life at room temperature
Dulbecco’s phosphate-buffered saline with calcium & magnesium (Gibco, catalog number: 14040-133), two years shelf life at room temperature
Matrigel (BD Biosciences, catalog number: 354234), two years of shelf life at -20 °C
Ketamine HCl (10 mg/mL) (COVETRUS, catalog number: 071069), two years shelf life at room temperature
Xylazine (1 mg/mL) (COVETRUS, catalog number: 061035), two years shelf life at room temperature
NYLON blue monofilament suture (TruStitch, catalog number: A662NV)
Ethiqa XR (Fidelis Pharmaceuticals LLC, catalog number: 86084-100-30), one year shelf life at room temperature
Paraformaldehyde (J.T. Baker, catalog number: S898-0), seven years of shelf life at room temperature
Silver nitrate (Sigma-Aldrich, catalog number: 10220-100G), >5 years shelf life at room temperature
4% PFA (see Recipes)
2% Nonidet P-40 (see Recipes)
0.02% NP40, 2% PFA (see Recipes)
5% sodium thiosulfate (see Recipes)
Anesthesia (see Recipes)
Equipment
Centrifuge (Thermo Scientific, Sorvall ST 16R Centrifuge)
Incubator (Thermo Forma, Series II Water Jacketed CO2 Incubator)
Procedure/Dissection Board (Aims Lab Products, GPM2)
Fiber-Optic Illuminator with dual goosenecks (Nikon)
Scissors (Iris scissors, Fine Science Tools)
Forceps (Dumont #5SF, Ring, and Graefe forceps, Fine Science Tools)
Needle holder: Halsted-Mosquito Hemostats (Fine Science Tools, catalog number: 91308-12)
Reflex Wound Clips (VWR, catalog number: 203-1000101326-476)
Stapler: Reflex Wound Clips (VWR, catalog number: 203-1000101326-476)
Electrocautery: Cautery Loop Tip High-Temperature 2200 °F (Bovie Medical Corporation)
Heat pad, microwavable (K&H Pet Products, catalog number: 69553)
Electric shaver (OSTER, catalog number: 078996-101-001)
Nikon stereoscope (NIKON, catalog number: SMZ1500)
Spot imaging system (Insight CMOS Digital Camera, SPOT)
Procedure
Preparation of primary suture cells for transplantation (102 to 105 cells)
Count cell numbers after suture cell isolation from mouse or human tissues.
Hint: Details for suture cell isolation can be found in the published protocol (Maruyama et al., 2022b).
Attention: Keep cells on ice.
Melt frozen Matrigel on ice.
Attention: Handle Matrigel on ice to prevent gelation before filling in a syringe.
Transfer the cell suspension from a 50 mL conical tube to a 1.5 mL microtube using a pipette.
Spin the cells down by centrifugation: 400 × g (400 rcf) for 7 min at 4 °C.
Carefully remove the supernatant from the cell pellet.
Hint: Gently tap the microtube to mix the cell pellet with the residual solution.
Resuspend the cells in 5 µL of Matrigel.
Fill the cell-embedded Matrigel (CEM) into an insulin syringe (Figure 1).
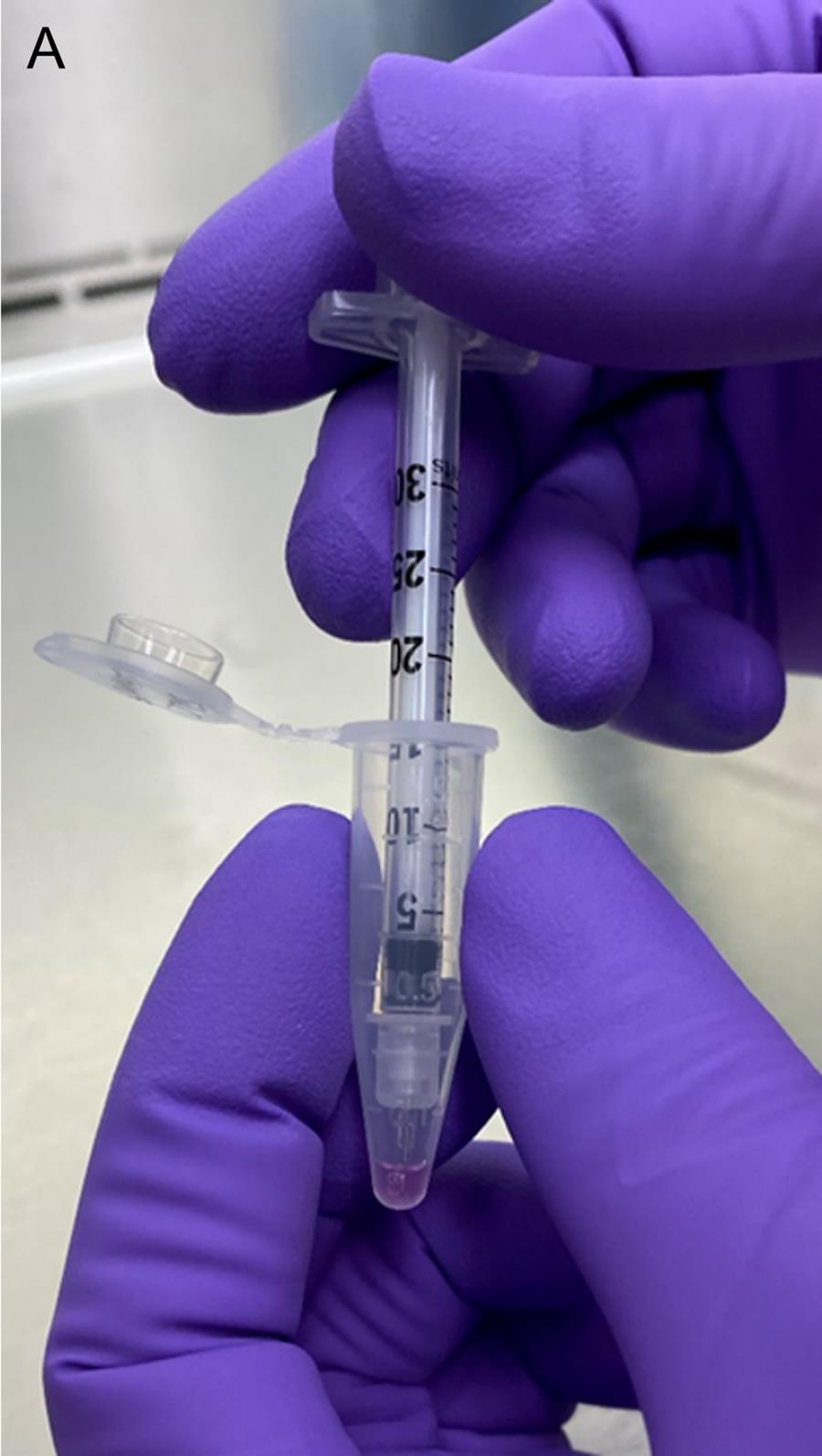
Figure 1. Filling the cell-embedded Matrigel into an insulin syringeIncubate the cell-filled syringe at 37°C for (at least) 5 min.
Hint: The CEM undergoes gelation at temperatures in the range of 22–35 °C.
Making the fire-polished glass capillary tube
Hold a glass capillary on both sides and apply heat at the center using a gas burner (Figure 2A).
Immediately after starting to feel the softness of the tube, gently pull the capillary tube toward both ends, forming a tip of approximately 0.2 mm in diameter.
Fire-polish the pulling side to smooth the tip (Figure 2B).
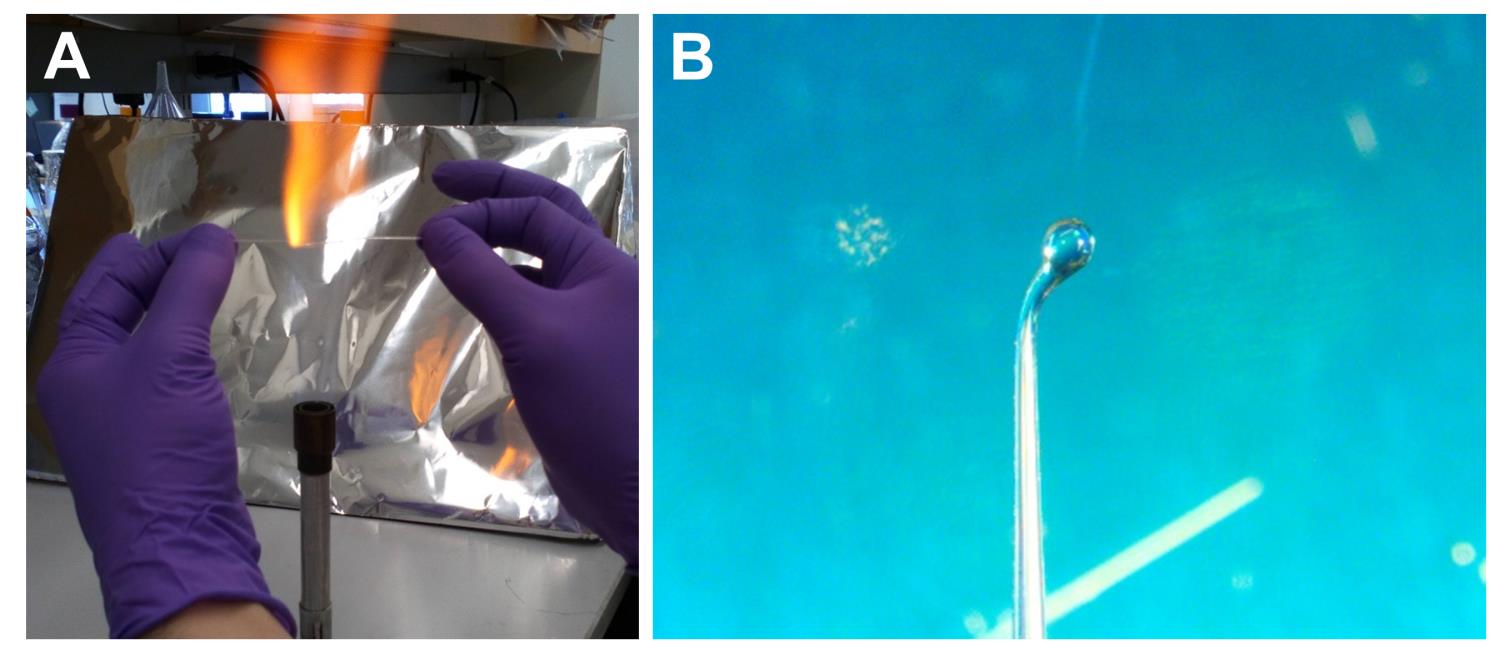
Figure 2. Preparation of the fire-polished capillary tube. (A) Pulling the capillary tube. (B) Fire polishing the pulling side.
Setup of the surgery field
Clean and sterilize the procedure table using 70% ethanol.
Assemble an area for surgery: a stage, light, an electrocautery device, a needle holder, a heating pad, and surgical tools, e.g., scissors, forceps, and staplers (Figure 3).
Attention: Surgical instruments are sterilized by autoclave.

Figure 3. Preparation of the operation field. The image shows an example of the surgery field.
Anesthesia and disinfection
Weigh the recipient SCID mouse to determine the dose of anesthetics: Ketamine and Xylazine.
Hint: Ketamine: 90–100 mg/kg (body weight) and Xylazine: 9–10 mg/kg (body weight).
Administer the anesthetic cocktail via intraperitoneal injection.
Transfer the animal to a sterile preoperative preparation bench.
Apply lubricating eye ointment to prevent dryness of the eyes during surgery (Figure 4A).
Shave back furs using the electric shaver and wipe the area with an alcohol pad (Figure 4B).
Disinfect the shaved area by gentle swabbing with an iodine solution (Figure 4C).
Wait a couple of minutes for disinfection (Figure 4D).
Remove the iodine by gentle swabbing with an alcohol pad (Figure 4E).
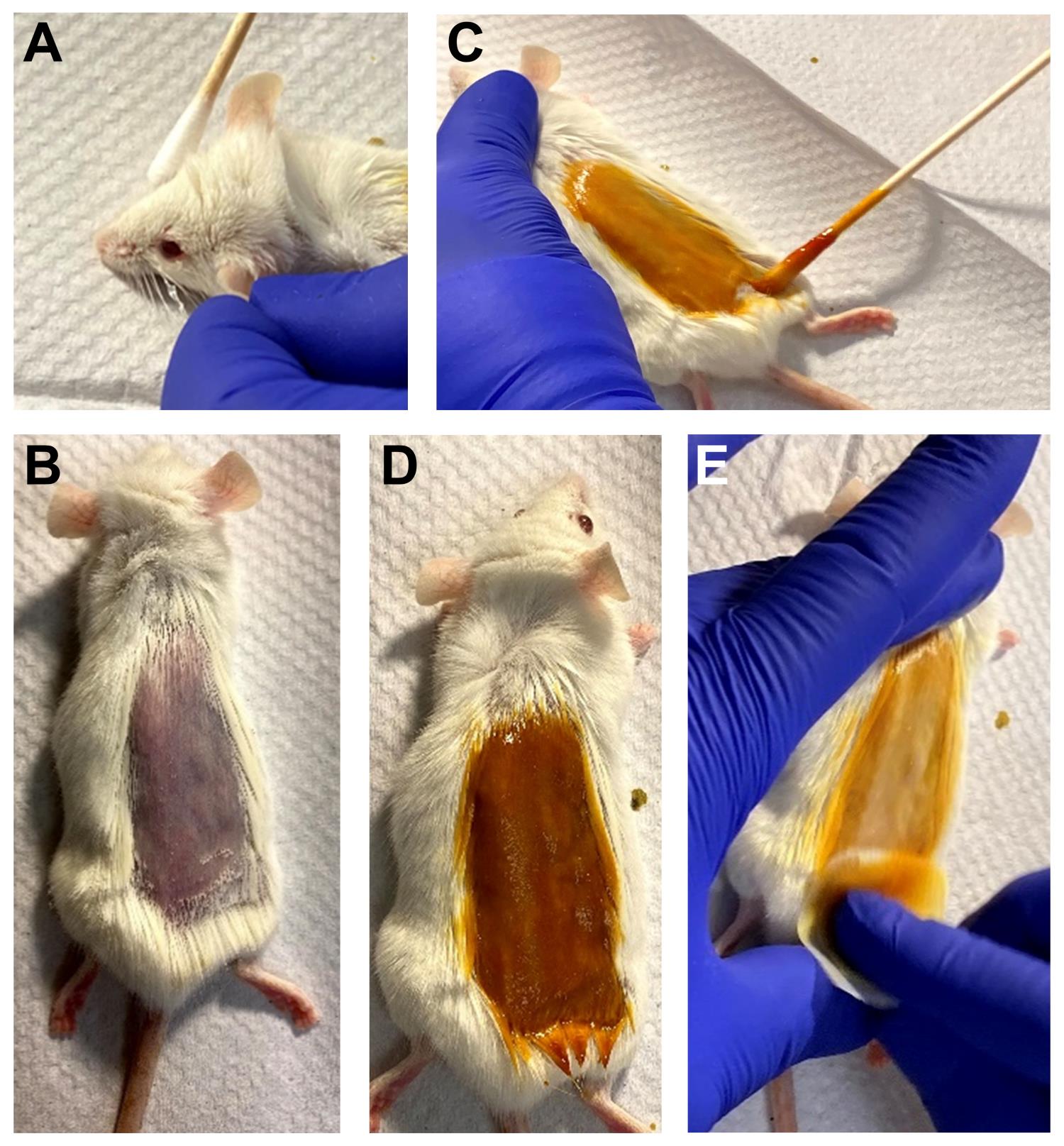
Figure 4. Preparation of animals for surgical operation. (A) Applying eye ointment. (B) Shaving the back furs. (C) Swabbing iodine. (D) Two minutes waiting. (E) Swabbing the iodine with an alcohol pad.
Transplantation
Lift the skin with forceps and use a pair of scissors to make a small incision (~1 cm) below the ribs and 0.5 cm off-center but parallel to the spine (Figure 5A).
Continue to cut through the peritoneum (Figure 5B).
Gently squeeze the abdomen under the incision to expose the kidney (Figure 5C).
Attention: Keep the kidney in a moist condition by applying PBS (Figure 5D).
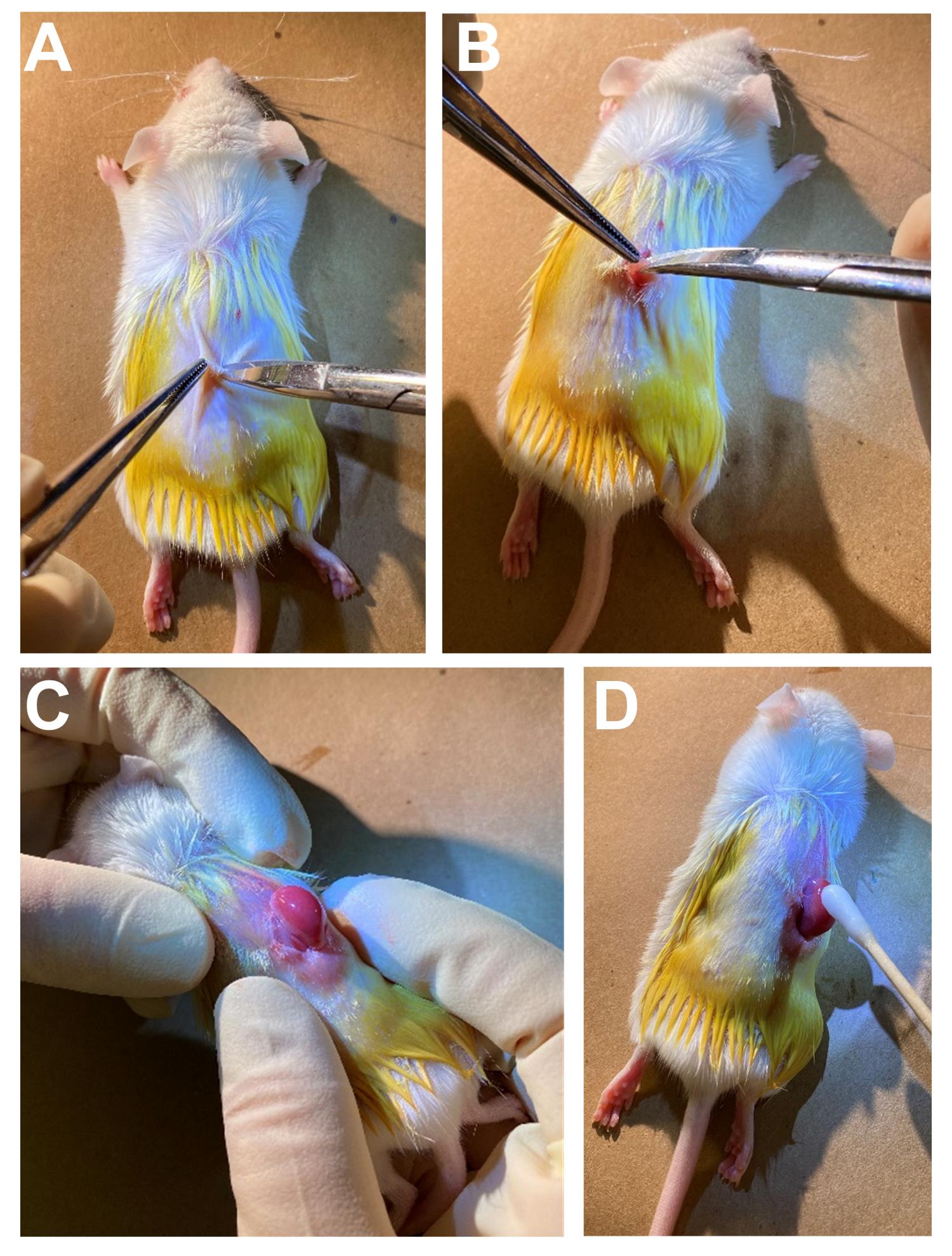
Figure 5. Preparation of animals for kidney transplantation. Incision of the skin (A), and the peritoneum (B). (C) Exposure of the kidney. (D) Apply moisture to the kidney.Carefully scratch the surface of the kidney with the needle to make a small opening at the injection site.
Gently insert the fire-polished tip of the capillary tube into the space between the kidney and outer membrane to make some room.
Hint: The idea is to see the opening to enable the syringe to easily penetrate the kidney capsule.
Hint: Do not damage the kidney; especially avoid significant bleeding, which would reduce transplantation efficacy.
Gently insert the insulin syringe containing CEM through the surface opening (Figure 6A).
Attention: The needle should go in a direction right underneath the outer membrane (kidney capsule region), but not into the kidney (Figure 6A’).
Inject the CEM underneath the kidney capsule (Figure 6B).
Hint: The CEM is visible and stable in the kidney capsule (Figure 6B’).
Close the small opening using electrocautery (Figure 6C).
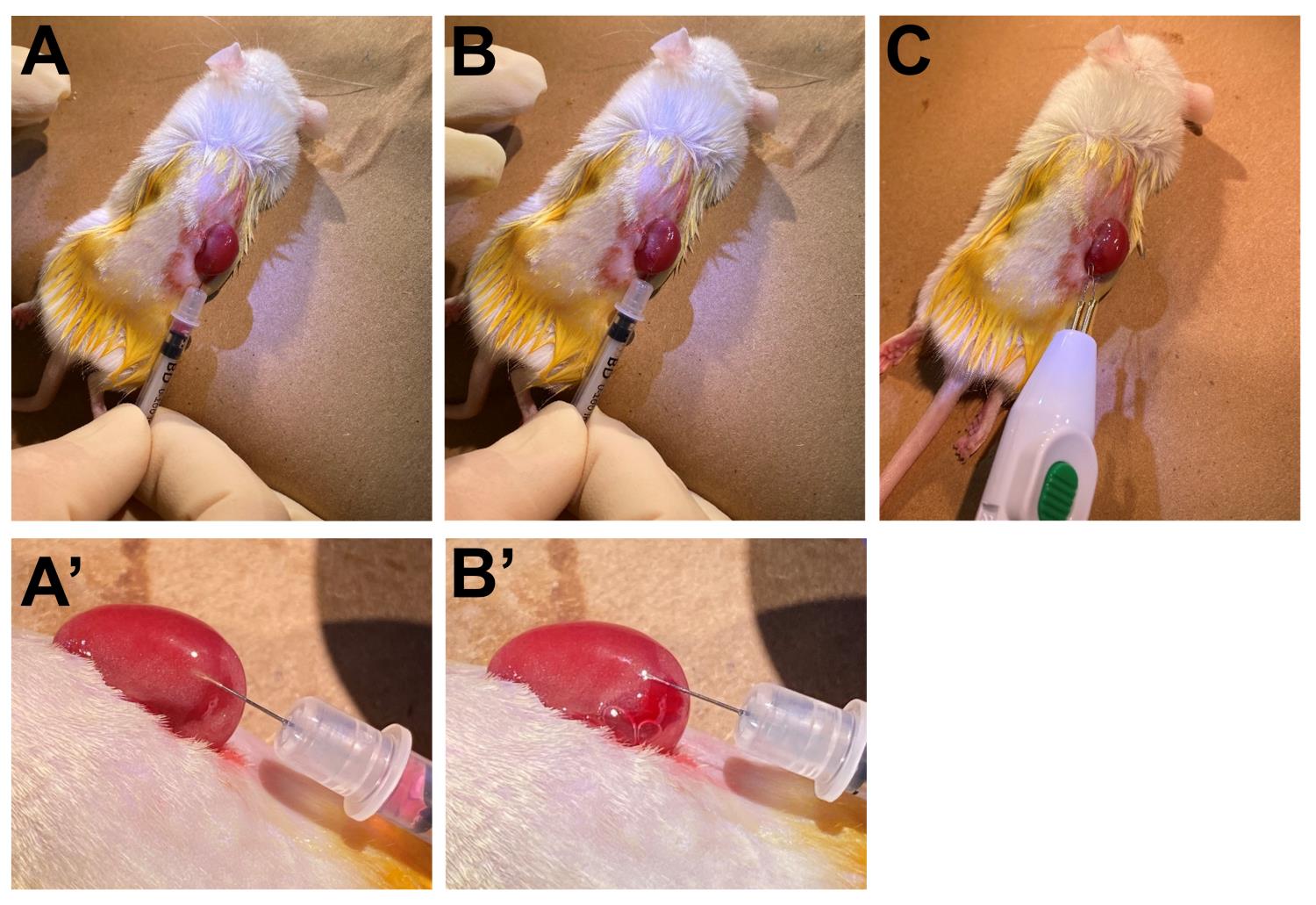
Figure 6. Implantation of cell-embedded Matrigel to the kidney capsule. (A, A’) Insertion of the syringe into the kidney capsule region. (B, B’) Injection of cell-embedded Matrigel. (C) Closure of the small opening.Gently move the kidney back to the abdominal cavity.
Close the peritoneum using absorbable polystyrene sutures (Figure 7A).
Close the skin using silk or nylon sutures (Figure 7B).
Hint: Alternatively, clip the sutured area using a stapler (Figure 7C).
Administer analgesics (e.g., Ethiqa) subcutaneously to mitigate the pain.
Transfer the animal to a clean heating pad to recover from anesthesia.
Monitor the animal continuously to observe any signs of labored breathing.
Once the animal starts to show signs of being responsive, transfer the animal to a clean cage, allowing free access to food and water.
Hint: There is no need to administer analgesic post-surgery because Ethiqa is effective up to 72 h.
Monitor the mouse daily for at least four consecutive days after surgery.
Retrieve and analyze the transplanted kidney at 2–4 weeks post-operation.
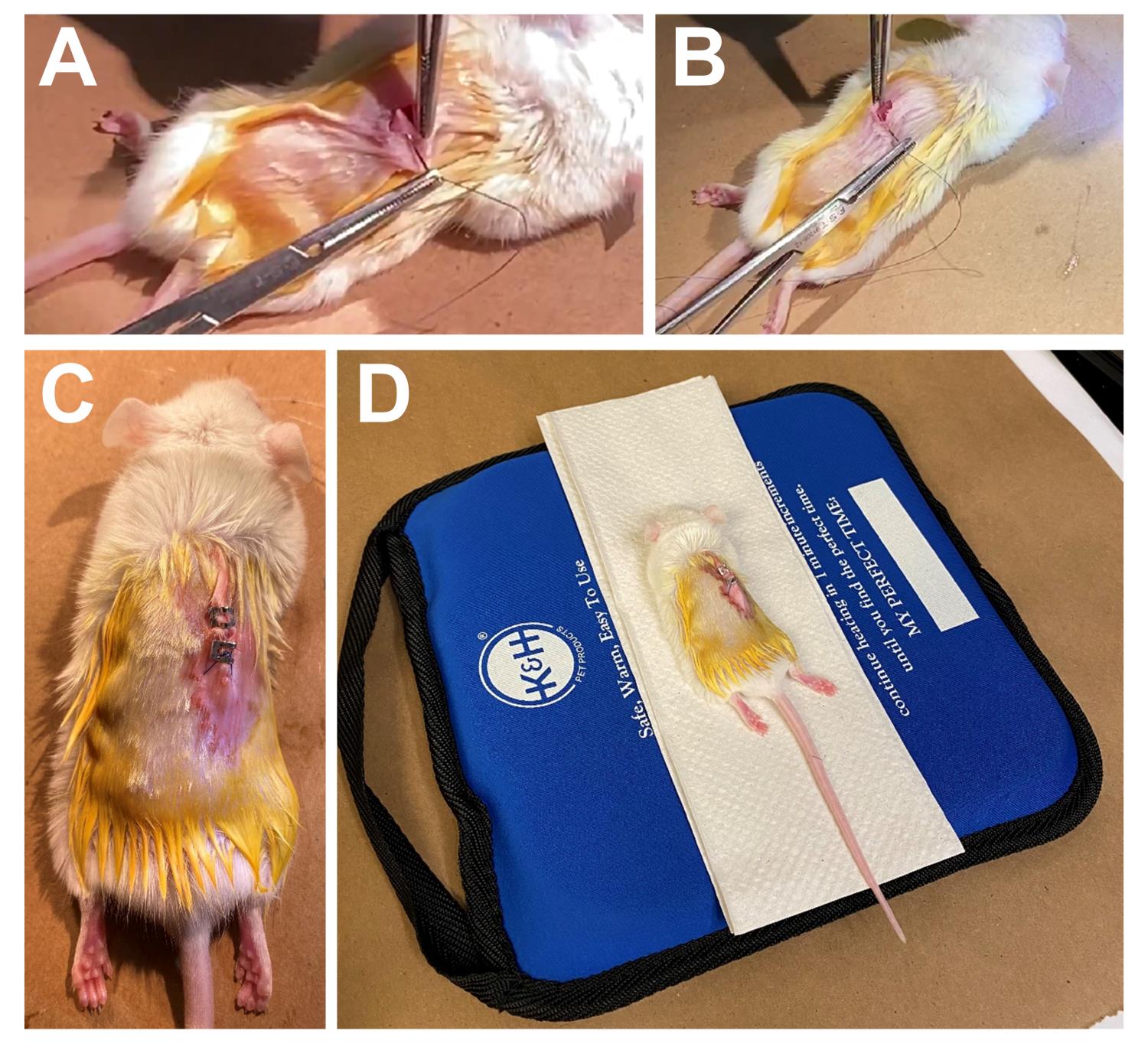
Figure 7. Post-surgical operation procedures. Closure of the peritoneum (A) and skin (B) and staple the sutured area (C).
Whole-mount von Kossa staining
Euthanize animals in a CO2 chamber.
Dissect the transplanted kidney and the contralateral kidney (as experimental control) and soak them in PBS on ice.
Photograph the transplant and control under the Nikon stereoscope using the SPOT image system (Figure 8A).
Prefix the transplant and control with 0.02% NP40/2% PFA in PBS at room temperature for 3 h.
Wash with distilled water at room temperature three times for 10 min each.
Hint: The washing process is critical to reduce background staining.
Place the sample in 1% aqueous silver nitrate at room temperature.
Expose the transplant and control under UV light for 20–60 min at room temperature.
Hint: Monitor the tissue every 10 min, until mineral deposition occurs and turns brown, to terminate the process.
Wash in distilled water at room temperature three times for 10 min each.
Place the stained kidney in 5% sodium thiosulfate at room temperature for 5 min.
Wash in distilled water at room temperature for 10 min.
Evaluate and photograph the stained transplant and control under the Nikon stereoscope using the SPOT imaging system (Figure 8B).
The sample can be post-fixed and decalcified for histological and immunological evaluations.
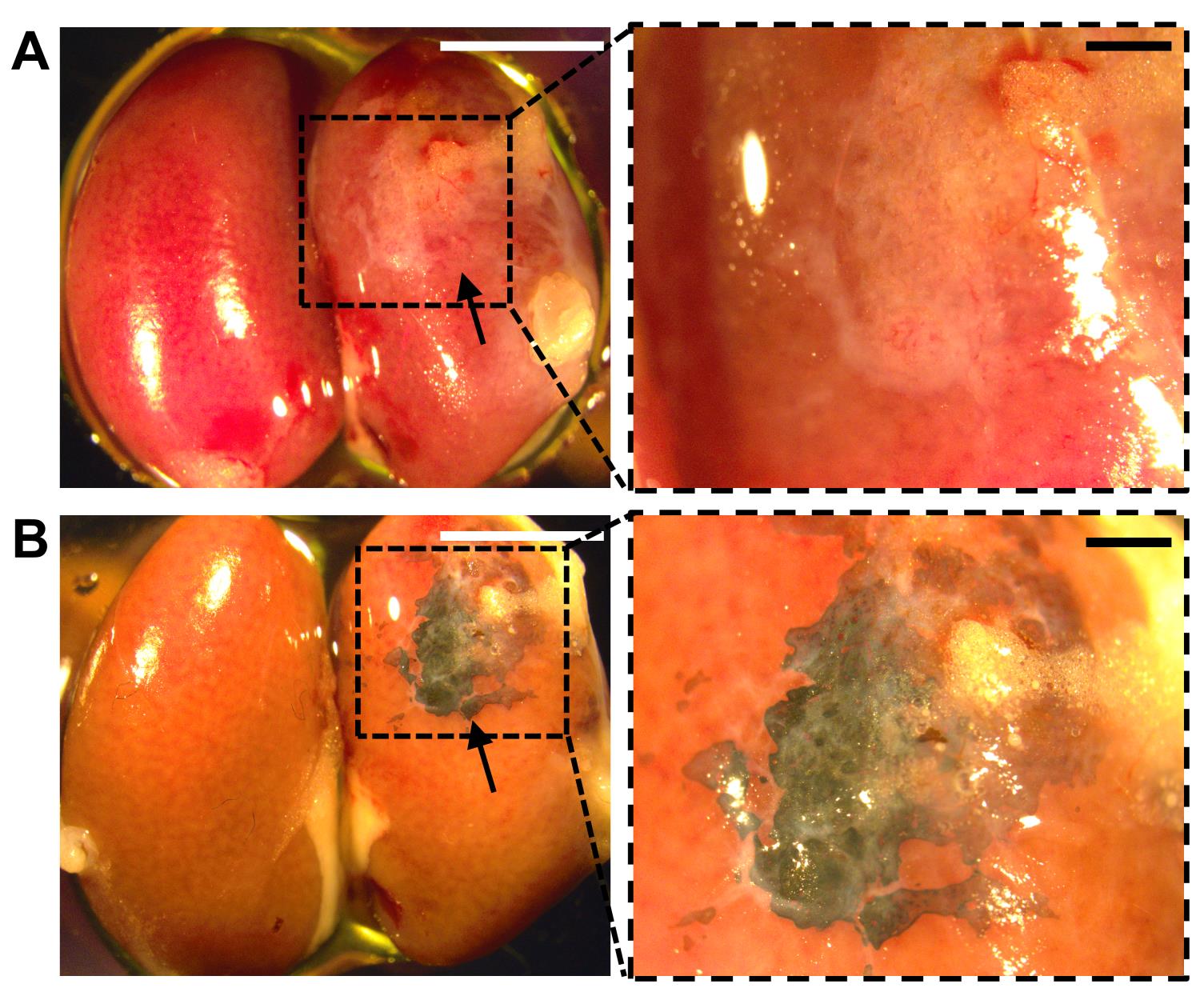
Figure 8. Analysis of transplanted kidney by whole mount von Kossa staining. Transplanted (right) and non-transplanted (left) kidneys before (A) and after (B) von Kossa staining. Arrows indicate the formation of ectopic bones at the kidney capsule. Enlargements of the inset in A and B are shown in a and b. Scale bars, 5 mm (A, B); 1 mm (a, b).
Data analysis
Stem cell frequency
Evaluation of bone formation after kidney capsule transplantation
Evaluate bone formation by whole-mount von Kossa staining of the transplanted kidney capsule using the Nikon stereoscope with the SPOT imaging system (Figures 4, 5, 6, 7, 8, Supplemental Figures S9, S10 of Maruyama et al., 2021).
Input data in a table format including dose (number of cells), tested (number of transplantations), and response (number of positive transplantations) to the ELDA software on the webpage (http://bioinf.wehi.edu.au/software/elda/).
Enter a confidence interval (95% as default) and select additional checkboxes if necessary.
Click on Run to determine stem cell frequency, based on the successful generation of bone in the transplants.
The “Estimate” indicates the stem cell frequency in the control and experimental groups.
Validate the likelihood ratio test for a single-hit model, to obtain the p-value for the determination of statistical significance (Figure 4 of Maruyama et al., 2021).
Recipes
4% PFA
40 g of paraformaldehyde in 1 L of PBS
2% NP 40
2 g of Nonidet P 40 in 100 mL of distilled water
0.02% NP40, 2% PFA
20 mL of 4% PFA
400 µL of 2% NP 40
19.6 mL of PBS
5% sodium thiosulfate
5 g of sodium thiosulfate in 100 mL of distilled water
Anesthesia
8.5 mL of DPBS (Dulbecco’s Phosphate-buffered Saline without calcium & magnesium)
1 mL of Ketamine (10 mg/mL)
0.5 mL of Xylazine (1 mg/mL)
Acknowledgments
The authors thank former and current lab members for their technical and intellectual support. This work is supported by the National Institutes of Health (DE15654, DE269369) and NYSTEM (C029558) to W.H. This protocol is derived from the original research papers published in Science Translational Medicine (Maruyama et al., 2021) and Science Advances (Maruyama et al., 2022a).
Competing interests
The authors declare no competing financial interests.
Ethics
The care and use of experimental animals described in this work comply with the guidelines and policies of IACUC at the Forsyth Institute and the University Committee on Animal Resources at the University of Rochester.
References
- Caplan, A. I. and Correa, D. (2011). The MSC: an injury drugstore. Cell Stem Cell 9(1): 11-15.
- Maruyama, T., Hasegawa, D., Valenta, T., Haigh, J., Bouchard, M., Basler, K. and Hsu, W. (2022a). GATA3 mediates nonclassical beta-catenin signaling in skeletal cell fate determination and ectopic chondrogenesis. Sci Adv 8(48): eadd6172.
- Maruyama, T., Jeong, J., Sheu, T. J. and Hsu, W. (2016). Stem cells of the suture mesenchyme in craniofacial bone development, repair and regeneration. Nature Commun 7: 10526.
- Maruyama, T., Stevens, R., Boka, A., DiRienzo, L., Chang, C., Yu, H. I., Nishimori, K., Morrison, C. and Hsu, W. (2021). BMPR1A maintains skeletal stem cell properties in craniofacial development and craniosynostosis. Sci Transl Med 13(583).
- Maruyama, T., Yu, H. I. and Hsu, W. (2022b). Skeletal Stem Cell Isolation from Cranial Suture Mesenchyme and Maintenance of Stemness in Culture. Bio Protoc 12(5): e4339.
- Robey, P. G., Kuznetsov, S. A., Riminucci, M. and Bianco, P. (2014). Bone marrow stromal cell assays: in vitro and in vivo. Methods Mol Biol 1130: 279-293.
- Sacchetti, B., Funari, A., Michienzi, S., Di Cesare, S., Piersanti, S., Saggio, I., Tagliafico, E., Ferrari, S., Robey, P. G., Riminucci, M., et al. (2007). Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131(2): 324-336.
- Scott, M. A., Levi, B., Askarinam, A., Nguyen, A., Rackohn, T., Ting, K., Soo, C. and James, A. W. (2012). Brief review of models of ectopic bone formation.Stem Cells Dev 21(5): 655-667.
- Takaoka, K., Nakahara, H., Yoshikawa, H., Masuhara, K., Tsuda, T. and Ono, K. (1988). Ectopic bone induction on and in porous hydroxyapatite combined with collagen and bone morphogenetic protein. Clin Orthop Relat Res (234): 250-254.
- Yang, Z., Yuan, H., Tong, W., Zou, P., Chen, W. and Zhang, X. (1996). Osteogenesis in extraskeletally implanted porous calcium phosphate ceramics: variability among different kinds of animals. Biomaterials 17(22): 2131-2137.
- Yu, P. B., Deng, D. Y., Lai, C. S., Hong, C. C., Cuny, G. D., Bouxsein, M. L., Hong, D. W., McManus, P. M., Katagiri, T., Sachidanandan, C., et al. (2008). BMP type I receptor inhibition reduces heterotopic [corrected] ossification.Nat Med 14(12): 1363-1369.
- Zeitouni, S., Krause, U., Clough, B. H., Halderman, H., Falster, A., Blalock, D. T., Chaput, C. D., Sampson, H. W. and Gregory, C. A. (2012). Human mesenchymal stem cell-derived matrices for enhanced osteoregeneration.Sci Transl Med 4(132): 132ra155.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Uchida, H., Maruyama, T. and Hsu, W. (2023). Determining Bone-forming Ability and Frequency of Skeletal Stem Cells by Kidney Capsule Transplantation and Limiting Dilution Assay. Bio-protocol 13(6): e4639. DOI: 10.21769/BioProtoc.4639.
- Maruyama, T., Hasegawa, D., Valenta, T., Haigh, J., Bouchard, M., Basler, K. and Hsu, W. (2022a). GATA3 mediates nonclassical beta-catenin signaling in skeletal cell fate determination and ectopic chondrogenesis. Sci Adv 8(48): eadd6172.
Category
Stem Cell > Adult stem cell > Cell transplantation
Developmental Biology > Morphogenesis > Organogenesis
Cell Biology > Cell Transplantation > Xenograft
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link