- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Multiphoton Microscopy of FITC-labelled Fusobacterium nucleatum in a Mouse in vivo Model of Breast Cancer
Published: Vol 13, Iss 6, Mar 20, 2023 DOI: 10.21769/BioProtoc.4635 Views: 1450
Reviewed by: Ivan ShapovalovAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantifying Bacterial Chemotaxis in Controlled and Stationary Chemical Gradients With a Microfluidic Device
Adam Gargasson [...] Harold Auradou
Feb 20, 2025 1710 Views

Optimized Protocol for the Collection, Cryopreservation, and In Vitro Cultivation of Human Gut Microbiota for Toxicomicrobiomics Applications
Paulina Średnicka [...] Michał Wójcicki
Nov 5, 2025 1951 Views

In Ovo CAM-Based Xenograft Model for Investigating Tumor Developmental Biology in Breast Cancer
Carlos César Patiño Morales [...] Ana Karen Herrera-Vargas
Feb 20, 2026 60 Views
Abstract
Over the past decades, the main techniques used to visualize bacteria in tissue have improved but are still mainly based on indirect recognition of bacteria. Both microscopy and molecular recognition are being improved, but most procedures for bacteria detection in tissue involve extensive damage. Here, we describe a method to visualize bacteria in tissue slices from an in vivo model of breast cancer. This method allows examining trafficking and colonization of fluorescein-5-isothiocyanate (FITC)-stained bacteria in various tissues. The protocol provides direct visualization of fusobacterial colonization in breast cancer tissue. Rather than processing the tissue or confirming bacterial colonization by PCR or culture, the tissue is directly imaged using multiphoton microscopy. This direct visualization protocol causes no damage to the tissue; therefore, all structures can be identified. This method can be combined with others to co-visualize bacteria, types of cells, or protein expression in cells.
Background
Fusobacterium nucleatum is an oral anaerobic bacterium that is prevalent in periodontal disease (Nozawa et al., 2020) and preterm birth (Hill, 1993, 1998; Han et al., 2009; Gonzales-Marin et al., 2013). Recently, F. nucleatum was found to colonize colorectal adenocarcinoma (Castellarin et al., 2012; Kostic et al., 2012), esophageal cancer (Yamamura et al., 2016), pancreatic cancer (Mitsuhashi et al., 2015), and breast cancer (Parhi et al., 2020). The presence of F. nucleatum in colon, pancreatic, and esophageal cancer has been associated with poor prognosis (Mima et al., 2016; Yamamura et al., 2016; Yamaoka et al., 2018; Mitsuhashi et al., 2015). In a mouse model, F. nucleatum colonizes breast cancer and promotes tumor growth and metastatic progression (Parhi et al., 2020).
In order to identify Fusobacterium nucleatum colonization in human and mouse cancer tissues, the methods previously used were microbiome analysis, PCR, fluorescence in situ hybridization, and culture (Castellarin et al., 2012; Kostic et al., 2012; Parhi et al., 2020; Abed et al., 2016; Abed et al., 2020). Specifically directing bacteria to a target cancer as a future therapy strategy requires visualization tools for imaging and monitoring bacterial trafficking in animal models. Here, we show a simple method that requires minimal time, equipment, materials, and resources to quickly validate bacterial localization in cancer tissue in an in vivo model. This is a direct method to visualize the bacteria in the tissue with minimal intermediate steps that may affect the results.
Materials and Reagents
1 mL syringe (BD PlastipakTM, catalog number: 303084)
27G and 26G needle (BD PlastipakTM, catalog numbers: 305109 and 303176)
Sterile surgical pad
10 cm tissue culture dish (Corning Incorporated, Falcon, catalog number: 353003)
11 mL (NuncTM catalog number: 347856), 15 and 50 mL tubes (Miniplast, catalog numbers: 835-015-40-111 and 835-050-21-111)
1.5 mL Eppendorf microtubes (Bar Naor Ltd., catalog number: BN022RL)
Scalpel (Bar Naor Ltd., catalog number: BN400-21-JH)
Whatman syringe filter 0.2 µm (Bar Naor Ltd., catalog number: BNFCA206030H)
Coverslip (Bar Naor Ltd., catalog number: BN1001-18-1CN)
Superfrost Plus glass slides (Thermo Fisher Scientific, catalog number: J1800AMNT)
Pipette tips [Axygen, catalog numbers: TF-200-R-S (20–200 μL), TF-1000-R-S (100–1,000 μL)]
Glass vials (Bar Naor Ltd., catalog number: BN1228WH)
Animals: 5–7-weeks-old female C57BL/6 mice (Envigo, Israel)
AT3 cell line (Sigma SCC178)
Dulbecco’s modified eagle medium (DMEM), 500 mL (Biological Industries, catalog number: 01-050-1A)
Accutase solution normal human, 100 mL (Biological Industries, catalog number: C-41310)
Dulbecco’s PBS, 500 mL (Biological Industries, catalog number: 02-020-1A)
Heat-inactivated fetal bovine serum (FBS), 500 mL (Biological Industries, catalog number: 04-127-1A)
L-glutamine in saline solution, 100 mL (Biological Industries, catalog number: 03-020-1B)
Penicillin–streptomycin solution, 100 mL (Biological Industries, catalog number: 03-031-1B)
MEM non-essential amino acids solution, 100 mL (Biological Industries, catalog number: 01-340-1B)
Fusobacterium nucleatum strain of interest (for example ATCC 23726)
Wilkins-Chalgren broth (Oxoid, catalog number: CM0643)
Fluorescein isothiocyanate (FITC), 1 g (Sigma-Aldrich, catalog number: F7250-1G)
Ethanol (Sigma-Aldrich, catalog number: E7023)
Trypan blue solution (Biological Industries, catalog number: 03-102-1B)
Sodium carbonate (Sigma-Aldrich, catalog number: 497-19-8)
Sodium hydroxide (Sigma-Aldrich, catalog number: 1310-73-2)
Isoflurane, USP TerrellTM (Piramal, catalog number: VED1350)
Double deionized water (DDW)
Ice
Wilkins medium (see Recipes)
Sodium carbonate buffer (0.1 M, pH 9.0) (see Recipes)
FITC solution (see Recipes)
Equipment
Pipettes
Centrifuge (Eppendorf 5810 R)
Autoclave (Tuttnauer 2540MK)
Anesthesia induction chamber
Hemocytometer
Spectrophotometer (600 nm wavelength)
Orbital shaker
Nikon multiphoton A1MP
Stereo microscope (Nikon SMZ2-)
Scalpel handle
Scissors
Forceps
Tail vein injection platform
Heat lamp
Anaerobic chamber (Bactron I–II Shellab, USA)
Caliper
Vortex
Software
NIS-Elements (Nikon Instruments Inc.) https://www.microscope.healthcare.nikon.com/products/software/nis-elements)
Procedure
Prepare cells
Seed 5 × 105 AT3 cells in a 10 cm tissue culture dish in 10 mL of DMEM medium containing 10% heat-inactivated FBS, 1% L-glutamine, 1% penicillin–streptomycin, and 1% MEM non-essential amino acids solution. Incubate the cells at 37 °C in a humidified incubator with 5% CO2 for 3–4 days. Ensure that the cells are 80%–100% confluent on the day of the experiment.
Change media (DMEM supplemented with 10% heat-inactivated FBS, 1% L-glutamine, 1% penicillin–streptomycin, and 1% MEM non-essential amino acids solution) the day before implantation.
To detach the tumor cells from the tissue culture dish, aspirate the culture medium, wash the cells with 5 mL of PBS, aspirate the PBS, and add 2 mL of accutase solution. Incubate the cells for 5–8 min with accutase at 37 °C. Trypsin/EDTA is an acceptable alternative to accutase.
Add 3 mL of DMEM to neutralize the accutase and pipette up and down until all cells have been detached. Transfer the cells suspension to a 15 mL conical centrifuge tube and add 10 mL of medium (the same as in step A1).
Centrifuge the cells at 1,000 rpm (200 × g) for 5 min at room temperature (RT). Aspirate the supernatant, leaving 500 μL of medium above the cell pellet. Resuspend the cells in the residual volume and add 10 mL of PBS. Invert the tube 2–3 times to get a homogeneous cell suspension and take 10 μL to count the cells in a hemocytometer. Use trypan blue to distinguish between live and dead cells.
Centrifuge the cell suspension at 200 × g for 5 min at RT. Aspirate the PBS and resuspend the cells in PBS at a final concentration of 2 × 106 cells/mL. Ensure that the single cell suspension has no clumping cells. Transfer the cells to an Eppendorf tube and keep on ice until use.
Orthotopical injection of AT3 tumor cells into mammary fat pad
Anesthetize the mice in an induction chamber receiving a slow flow rate of isoflurane (3%–5% in oxygen).
Place the mouse on a sterile surgical pad and make sure the head is properly placed inside the isoflurane nose cone. Confirm proper anesthesia by pinching the paw. Disinfect the injection site with 70% ethanol. Consider applying eye gel to prevent vision deterioration while the mouse is anesthetized.
Inject subcutaneously 1 × 106 AT3 cells in 50 μL of PBS orthotopically into the inguinal mammary fat pad using a 1 mL syringe with a 26G needle, by raising the nipple with forceps and inserting the needle underneath and perpendicular to the nipple.
Monitor the mice until they regain consciousness; make sure they regain full consciousness before joining other mice.
Bacteria growth, staining, and injection to mice
Perform bacterial injection when tumor size reaches 500 mm3 measured externally by caliper.
Grow F. nucleatum strain of interest in 5 mL of sterile Wilkins medium (see Recipes) overnight for 12–18 h in an anaerobic chamber in an atmosphere of 90% N2, 5% CO2, and 5% H2 at 37 °C.
Centrifuge at 2,057 × g for 5 min at 4 °C and discard supernatant. Resuspend the pellet with 1 mL of PBS, then add 4 mL of PBS and centrifuge again under the same conditions.
Discard the supernatant and resuspend the pellet with 1 mL of a fresh FITC solution (see Recipes). Ensure that the single cell suspension has no clumps.
Incubate in the dark with orbital rotation for 30 min at RT.
Add 4 mL of PBS and suspend. Centrifuge at 2,057 × g for 5 min at 4 °C and repeat the washing until the PBS in the supernatant is clear.
Discard the supernatant, resuspend the bacteria with 1 mL of PBS, and bring the bacteria to an OD600 of 1 (approximately 1 × 109 bacteria/mL). Keep bacteria on ice and in the dark until use.
Restrain the mouse in a tail vein injection platform. Position the tail such that the vein is facing upwards.
Prior to injection, warm the tail using an overhead heat lamp for a few minutes to dilate the veins.
Using a 1 mL syringe and a 27G needle, insert the needle gently, while the bevel is up, into the lateral or dorsal tail vein towards the direction of the head. Keep the needle and syringe parallel to the tail. Inject intravenously 5 × 107 fluorescein-5-isothiocyanate (FITC)-labeled F. nucleatum ATCC 23726. The contents of the needle should inject easily without resistance. In order to confirm correct intravenous injection, the piston should easily move and the vein color should change to a whiter color momentaneously.
Mice sacrifice and tissue harvesting
Twenty-four hours after bacterial injection, transfer mice to a chamber containing 2% isoflurane in oxygen for approximately 30 s.
Verify that mice are fully anesthetized and perform cervical dislocation. Another option is performing CO2 euthanasia followed by confirmation by cervical dislocation.
Clean tumor area with 70% ethanol. Cut the skin with a pair of sterilized scissors in the ventral middle of the mice, near the tumor. Hold the skin near the tumor with sterile forceps and remove tumor carefully using a sterile scalpel.
Hold the skin on the other side of the mice’s body near the healthy (control) breast tissue with sterile forceps and remove tissue carefully using a sterilized scalpel.
Place tumor and healthy breast tissue in a glass vial containing PBS, on ice.
Multiphoton microscopy of FITC-labeled F. nucleatum
Cut a slice from the fresh tissue using a scalpel and photograph using a Nikon SMZ25. Representative images are shown in Figure 1.
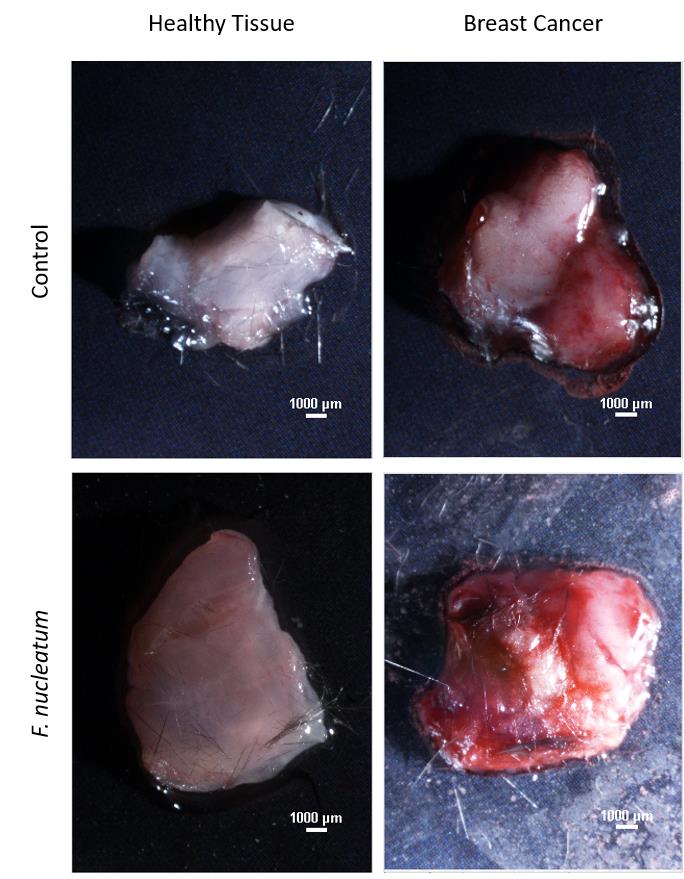
Figure 1. Nikon SMZ25 images of breast cancer tissue and adjacent mammary tissue. Representative microscopy images of tumor and normal adjacent mammary samples harvested 24 h post intravenous injection of 5 × 107 FITC-labeled F. nucleatum ATCC 23726 or with PBS vehicle into AT3 cancer-bearing mice.Place the tissue on a slide with a drop of water and cover with a coverslip.
Observe and photograph immunofluorescent images using the Nikon multiphoton A1MP microscope set to 740 nm wavelength using a 25× objective. Representative images are shown in Figure 2.
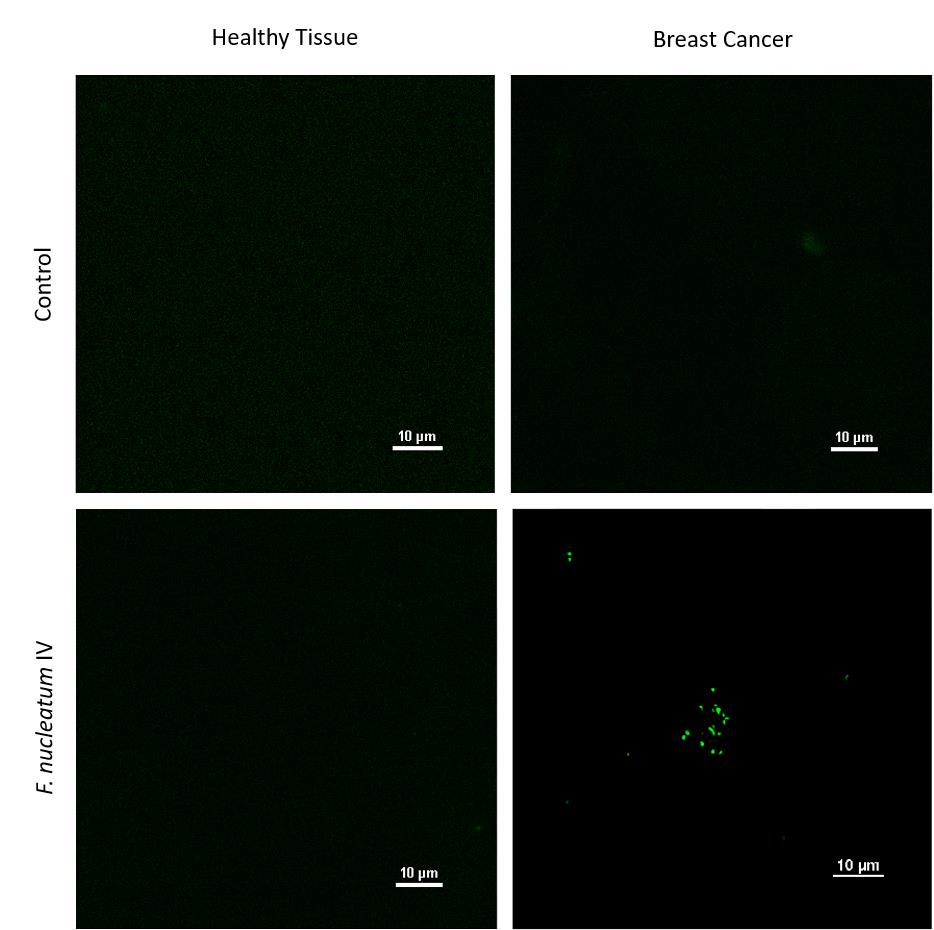
Figure 2. Nikon multiphoton A1MP microscope images of breast cancer tissue and adjacent mammary tissue. Representative multiphoton microscopy images of tumor and normal adjacent mammary samples harvested 24 h post intravenous injection of AT3 cancer-bearing mice with 5 × 107 FITC-labeled F. nucleatum ATCC 23726. Images were taken from Parhi et al. (2020). No changes were made.
Data analysis
Images obtained from the fluorescence microscope are converted to 8-bit images by NIS-Elements software (see Figures 1 and 2).
Recipes
Wilkins medium
100 mL of DDW
3.1 g of Wilkins-Chalgren broth
Mix until dissolved
Autoclave at 121 °C for 30 min and aliquot 5 mL to 11 mL tube
Reduce in an anaerobic chamber overnight
Sodium carbonate buffer (0.1 M, pH 9.0)
84 mg of sodium carbonate
10 mL of DDW
Adjust pH to 9.0 with sodium hydroxide
Sterilize by filtering through 0.2 μm Whatman filter
FITC solution
Dissolve 1 mg of FITC in 10 mL of sodium carbonate buffer by vortexing
Keep in the dark until used
Acknowledgments
The authors thank Dr. Zakhariya Manevitch for his valuable help in microscopy. This work was supported by the Israel Cancer Research Fund Project grant and the Israel Science Foundation Moked grant. This protocol was derived from Parhi et al. (2020). Nature Communications. https://doi.org/10.1038/s41467-020-16967-2
Competing interests
The authors declare no competing interests.
Ethics
Experiments were carried out under protocol MD-17-15239-5 approved by the Hebrew University of Jerusalem Ethics Committee and signed by the chairperson Prof. Sara Eyal.
References
- Abed, J., Emgard, J. E., Zamir, G., Faroja, M., Almogy, G., Grenov, A., Sol, A., Naor, R., Pikarsky, E., Atlan, K. A., et al. (2016). Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 20(2): 215-225.
- Abed, J., Maalouf, N., Manson, A. L., Earl, A. M., Parhi, L., Emgard, J. E. M., Klutstein, M., Tayeb, S., Almogy, G., Atlan, K. A., Chaushu, S., Israeli, E., Mandelboim, O., Garrett, W. S. and Bachrach, G. (2020). Colon Cancer-Associated Fusobacterium nucleatum May Originate From the Oral Cavity and Reach Colon Tumors via the Circulatory System. Front Cell Infect Microbiol 10: 400.
- Castellarin, M., Warren, R. L., Freeman, J. D., Dreolini, L., Krzywinski, M., Strauss, J., Barnes, R., Watson, P., Allen-Vercoe, E., Moore, R. A., et al. (2012). Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 22(2): 299-306.
- Gonzales-Marin, C., Spratt, D. A. and Allaker, R. P. (2013). Maternal oral origin of Fusobacterium nucleatum in adverse pregnancy outcomes as determined using the 16S-23S rRNA gene intergenic transcribed spacer region. J Med Microbiol 62(Pt 1): 133-144.
- Han, Y. W., Shen, T., Chung, P., Buhimschi, I. A. and Buhimschi, C. S. (2009). Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol 47(1): 38-47.
- Hill, G. B. (1993). Investigating the source of amniotic fluid isolates of fusobacteria. Clin Infect Dis 16 Suppl 4: S423-424.
- Hill, G. B. (1998). Preterm birth: associations with genital and possibly oral microflora. Ann Periodontol 3(1): 222-232.
- Mima, K., Nishihara, R., Qian, Z. R., Cao, Y., Sukawa, Y., Nowak, J. A., Yang, J., Dou, R., Masugi, Y., Song, M., et al. (2016). Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65(12): 1973-1980.
- Mitsuhashi, K., Nosho, K., Sukawa, Y., Matsunaga, Y., Ito, M., Kurihara, H., Kanno, S., Igarashi, H., Naito, T., Adachi, Y., et al. (2015). Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 6(9): 7209-7220.
- Nozawa, A., Oshima, H., Togawa, N., Nozaki, T. and Murakami, S. (2020). Development of Oral Care Chip, a novel device for quantitative detection of the oral microbiota associated with periodontal disease. PLoS One 15(2): e0229485.
- Kostic, A. D., Gevers, D., Pedamallu, C. S., Michaud, M., Duke, F., Earl, A. M., Ojesina, A. I., Jung, J., Bass, A. J., Tabernero, J., et al. (2012). Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22(2): 292-298.
- Parhi, L., Alon-Maimon, T., Sol, A., Nejman, D., Shhadeh, A., Fainsod-Levi, T., Yajuk, O., Isaacson, B., Abed, J., Maalouf, N., et al. (2020). Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat Commun 11(1): 3259.
- Yamamura, K., Baba, Y., Nakagawa, S., Mima, K., Miyake, K., Nakamura, K., Sawayama, H., Kinoshita, K., Ishimoto, T., Iwatsuki, M., et al. (2016). Human Microbiome Fusobacterium nucleatum in Esophageal Cancer Tissue Is Associated with Prognosis. Clin Cancer Res 22(22): 5574-5581.
- Yamaoka, Y., Suehiro, Y., Hashimoto, S., Hoshida, T., Fujimoto, M., Watanabe, M., Imanaga, D., Sakai, K., Matsumoto, T., Nishioka, M., et al. (2018). Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J Gastroenterol 53(4): 517-524.
Article Information
Copyright
© 2023 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Parhi, L., Shhadeh, A., Maalouf, N., Alon-Maimon, T., Scaiewicz, V. and Bachrach, G. (2023). Multiphoton Microscopy of FITC-labelled Fusobacterium nucleatum in a Mouse in vivo Model of Breast Cancer. Bio-protocol 13(6): e4635. DOI: 10.21769/BioProtoc.4635.
Category
Cancer Biology > General technique > Animal models
Biological Sciences > Microbiology > Microbial communities
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









