- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
High-throughput Assessment of Mitochondrial Protein Synthesis in Mammalian Cells Using Mito-FUNCAT FACS
Published: Vol 13, Iss 3, Feb 5, 2023 DOI: 10.21769/BioProtoc.4602 Views: 1864
Reviewed by: Gal HaimovichBrian M. ZidAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Novel Antibody-independent Method to Measure Complement Deposition on Bacteria
Toska Wonfor [...] Maisem Laabei
May 5, 2023 1480 Views

Immunofluorescent Staining Assay of 3D Cell Culture of Colonoids Isolated from Mice Colon
Trisha Mehrotra [...] Didier Merlin
Mar 5, 2024 2479 Views

Intraepidermal Nerve Fiber Quantification of the Mouse Hind Paw Footpads: A Detailed and Simplified Protocol
Anastasia Yerushkin [...] Amir Dori
Dec 5, 2025 1214 Views
Abstract
In addition to cytosolic protein synthesis, mitochondria also utilize another translation system that is tailored for mRNAs encoded in the mitochondrial genome. The importance of mitochondrial protein synthesis has been exemplified by the diverse diseases associated with in organello translation deficiencies. Various methods have been developed to monitor mitochondrial translation, such as the classic method of labeling newly synthesized proteins with radioisotopes and the more recent ribosome profiling. However, since these methods always assess the average cell population, measuring the mitochondrial translation capacity in individual cells has been challenging. To overcome this issue, we recently developed mito-fluorescent noncanonical amino acid tagging (FUNCAT) fluorescence-activated cell sorting (FACS), which labels nascent peptides generated by mitochondrial ribosomes with a methionine analog, L-homopropargylglycine (HPG), conjugates the peptides with fluorophores by an in situ click reaction, and detects the signal in individual cells by FACS equipment. With this methodology, the hidden heterogeneity of mitochondrial translation in cell populations can be addressed.
Keywords: MitochondriaBackground
Mitochondria are important power plant organelles that produce ATP through oxidative phosphorylation (OXPHOS). Since the symbiosis of the bacterial ancestor, the mitochondrial genome has been minimized due to gene transfer to the nuclear genome. In humans, the organelle genome contains only 13 mRNAs, which all encode a subunit of OXPHOS complexes(Anderson et al., 1981). Despite the small number of mRNAs, their translation by mitochondrial ribosomes (mitoribosomes) is essential since defects in organello translation lead to several diseases, such as mitochondrial myopathy, encephalopathy, lactic acidosis, stroke-like episodes, and myoclonus epilepsy associated with ragged-red fibers (Gorman et al., 2016).
Therefore, mitochondrial translation has been researched by several methods [see Apostolopoulos and Iwasaki (2022) for a summary], such as35S-methionine-labeling of newly synthesized nascent chains (Chomyn, 1996;Sasarman and Shoubridge, 2012), the quantification of ribosome-associated mRNAs by quantitative reverse transcription-PCR (Antonicka et al., 2013; Fung et al., 2013; Zhang et al., 2014; Pearce et al., 2017), ribosome profiling (Rooijers et al., 2013; Iwasaki et al., 2016; Gao et al., 2017; Pearce et al., 2017; Morscher et al., 2018; Suzuki et al., 2020; Kashiwagi et al., 2021; Li et al., 2021; Schöller et al., 2021), and pulse stable isotope labeling by amino acids in cell culture for proteomic analysis (Imami et al., 2021). However, these approaches provide averaged data for thousands of cells, which are pooled for the experiments, and thus pose an analytic hurdle when assessing mitochondrial translation in each individual cell.
However, an alternative method may overcome this barrier. In this technique, click-reaction-compatible methionine analogs, such as L-homopropargylglycine (HPG) and L-azidohomoalanine (AHA), are employed for nascent chain labeling. Subsequent fluorophore conjugation with the click reaction monitors mitochondrial protein synthesis (fluorescent noncanonical amino acid tagging, FUNCAT) (Dieterich et al., 2010). By shutting off cytosolic translation with compounds (such as anisomycin), only the proteins synthesized in the mitochondria (mito-FUNCAT) are labeled (Zhang et al., 2014; Estell et al., 2017; Yousefi et al., 2021; Zorkau et al., 2021). In addition to the in vitro click reaction and subsequent detection on a gel (on-gel mito-FUNCAT), in situ fluorophore conjugation provides visualization of mitochondrial protein synthesis in each cell under a microscope (in situ mito-FUNCAT) (Zhang et al., 2014; Estell et al., 2017;Yousefi et al., 2021;Zorkau et al., 2021).
Recently, we further modified the in situ mito-FUNCAT to perform high-throughput measurements by applying fluorescence-activated cell sorting (FACS) on a massive number of cells (mito-FUNCAT FACS) (Kimura et al., 2022). In this manuscript, we describe the step-by-step protocol of this method, which is applicable to various cell lines. We added Cy3 to HPG-labeled polypeptides and simultaneously stained Tom20 with Alexa-Fluor 647 (AF 647) by immunostaining. Given that the abundance of Tom20 represents the mitochondrial mass and allows mitochondrial protein synthesis to be normalized, this method ensures that the alteration of synthesized protein originates from mitochondrial biogenesis or net translation. Moreover, with mito-FUNCAT FACS, the unexpected cellular heterogeneity of mitochondrial translation in cell populations can be determined. The application of this method will provide pivotal insights into the regulatory mechanisms of mitochondrial protein synthesis across cell types, development, stress response, etc.
Materials and Reagents
Falcon 25 cm2 rectangular canted neck cell culture flask with plug seal screw cap (Corning, catalog number: 353082)
Nunc EasYDish dishes 100 mm (Thermo Fisher Scientific, catalog number: 150466)
Primaria 25 cm2 rectangular canted neck cell culture flask with vented cap (Corning, catalog number: 353808)
Safe lock tubes 2.0 mL (Eppendorf, catalog number: 3-7353-04)
Tube, 15 mL, PP, 17/120 mm, conical bottom, cellstar, blue screw cap, natural, graduated, writing area, sterile, 5 psc./bag, triple packed (Greiner Bio-One, catalog number: 188271)
Pipette, 10 mL, graduated 1/10 mL, sterile, paper-plastic packaging, single packed (Greiner Bio-One, catalog number: 607180)
Pipette, 25 mL, graduated 2/10 mL, sterile, paper-plastic packaging, single packed (Greiner Bio-One, catalog number: 760180)
10 μL, long filter tip, graduated, system rack (PP), sterilized (WATSON, catalog number: 1252P-207CS)
20 μL, hyper filter tip, refill plate, sterilized (WATSON, catalog number: 127-20S)
200 μL, hyper filter tip, refill plate, sterilized (WATSON, catalog number: 0127-20S)
1,000 μL, long filter tip, graduated, refill plate, sterilized (WATSON, catalog number: 126-1000S)
Falcon 5 mL round bottom polystyrene test tube, with snap cap, sterile (Corning, catalog number: 352058)
Falcon 5 mL round bottom polystyrene test tube, with cell strainer snap cap (Corning, catalog number: 352235)
Stericup quick release-GP sterile vacuum filtration system (Millipore, catalog number: S2GPU01RE)
A375 [American Type Culture Collection (ATCC), catalog number: CRL-1619]
C2C12 (ATCC, catalog number: CRL-1772)
H1944 (ATCC, catalog number: CRL-5907)
H2009 (ATCC, catalog number: CRL-5911)
H2122 (ATCC, catalog number: CRL-5985)
H441 (ATCC, catalog number: HTB-174)
HEK293 (ATCC, catalog number: CRL-1573)
HEK293T (ATCC, catalog number: CRL-3216)
HeLa S3 (RIKEN BioResource Research Center, RCB1525)
Alexa-Fluor 647 Anti-Tom20 antibody (Abcam, catalog number: ab209606, stored at 4°C)
Anisomycin (from Streptomyces griseolus) (Chem-Impex International, catalog number: 00466, stored at -20°C)
10× Click-iT cell reaction buffer (Thermo Fisher Scientific, catalog number: C10269, stored at 4°C)
Click-iT reaction buffer additive (Thermo Fisher Scientific, catalog number: C10269, stored at -20°C)
Copper (II) sulfate (CuSO4) (Thermo Fisher Scientific, catalog number: C10269, stored at 4°C)
Cy3-azide (Jena Bioscience, catalog number: CLK-046, stored at -20°C)
Digitonin (Nacalai Tesque, catalog number: 19390-91, stored at room temperature)
DMEM, high glucose, GlutaMAX supplement (Thermo Fisher Scientific, catalog number: 10566-016, stored at 4°C)
DMEM, high glucose, no glutamine, no methionine, no cystine (Thermo Fisher Scientific, catalog number: 21013-024, stored at 4°C)
Dimethyl sulfoxide (DMSO), nuclease and protease tested (Nacalai Tesque, catalog number: 09659-14, stored at room temperature)
L-homopropargylglycine (HPG) (Jena Bioscience, catalog number: CLK-1067, stored at -20°C)
Fetal bovine serum (FBS) (Sigma-Aldrich, catalog number: F7524, stored at -20°C)
1 mol/L HEPES-KOH buffer solution (pH 7.5) (Nacalai Tesque, catalog number: 15639-84, stored at room temperature)
Intercept (TBS) blocking buffer (LI-COR, catalog number: 927-60001, stored at 4°C)
200 mmol/L L-alanyl-L-glutamine solution (100×) (Nacalai Tesque, catalog number: 04260-64, stored at 4°C)
L-cystine dihydrochloride, animal-free (Nacalai Tesque, catalog number: 13003-12, stored at room temperature)
1 mol/L magnesium chloride solution (MgCl2), sterile-filtered (Nacalai Tesque, catalog number: 20942-34, stored at room temperature)
4% paraformaldehyde phosphate buffer solution (Nacalai Tesque, catalog number: 09154-85, stored at 4°C)
5 mol/L sodium chloride solution (NaCl) (Nacalai Tesque, catalog number: 06900-14, stored at room temperature)
Sucrose, ultra-pure (Wako, catalog number: 198-13525, stored at room temperature)
Triton X-100 (Nacalai Tesque, catalog number: 12967-32, stored at room temperature)
Trypsin-EDTA (0.05%), phenol red (Thermo Fisher Scientific, catalog number: 25300-054, stored at 4°C)
UltraPure DNase/RNase-Free distilled water (Thermo Fisher Scientific, catalog number: 10977-015, stored at room temperature)
Bovine serum albumin (Sigma-Aldrich, catalog number: A4919)
Met-free DMEM (500 mL) (see Recipes)
100 mg/mL anisomycin (250 µL) (see Recipes)
100 mM HPG (6.11 mL) (see Recipes)
Met-free DMEM with anisomycin and HPG (10 mL) (see Recipes)
Met-free DMEM with anisomycin (10 mL) (see Recipes)
0.5% digitonin (10 mL) (see Recipes)
Mitochondria-protective buffer (5 mL) (see Recipes)
Mitochondria-protective buffer with 0.0005% digitonin (5 mL) (see Recipes)
10% Triton X-100 (40 mL) (see Recipes)
PBS with 0.1% Triton X-100 (0.5 mL) (see Recipes)
5 mM Cy3-azide (370 µL) (see Recipes)
50 µM Cy3-azide (100 µL) (see Recipes)
Click-reaction mixture (1 mL) (see Recipes)
Intercept (TBS) blocking buffer with Alexa-Fluor 647 Anti-Tom20 antibody (101 µL) (see Recipes)
FACS buffer (see Recipes)
Equipment
High-speed micro centrifuge (himac, model: CF16RN)
High-speed refrigerated micro centrifuge (TOMY, model: MDX-310)
Swing rotor (himac, model: T4SS31)
Swing rack (TOMY, model: SAR015-24)
CO2 incubator (PHCbi, model: MAC-170AIC)
Pipet-Aid XP2 (Drummond Scientific Company, catalog number: 4-040-501)
Pipetman P-10 (Gilson, catalog number: F144802)
Pipetman P-20 (Gilson, catalog number: F123600)
Pipetman P-200 (Gilson, catalog number: F123601)
Pipetman P-1000 (Gilson, catalog number: F123602)
BD FACSMelody [BD, 3-laser (488, 640, and 561 nm), 8-color (2-2-4) configuration)]
CellDrop BF (DeNovix, CellDrop BF-UNLTD)
Software
BD FACSChorus (BD FACSMelody operating software)
FlowJo (BD, v10.8.1)
R version 4.1.1 (R Development Core Team, https://www.r-project.org )
Procedure
Note: When the cells are centrifuged, use swing rotors.
Cell culture
Culture cells to 70%–80% confluency with 5 mL of medium in a humidified incubator with 5% CO2 at 37°C. The media and flask used for each cell line in our experiments are described below.
Medium and flasks:
Cell type Media Flask C2C12 DMEM, high glucose, GlutaMAX supplement with 10% FBS Falcon T25 flask HeLa S3 Falcon T25 flask HEK293 Falcon T25 flask HEK293T Falcon T25 flask A375 Falcon T25 flask H2009 Falcon T25 flask H2122 RPMI 1640 medium with 10% FBS Primaria T25 flask H1944 Falcon T25 flask H441 Falcon T25 flask Aspirate the medium from the flask and add 3 mL of PBS slowly.
Aspirate PBS from the flask and add 1 mL of trypsin-EDTA.
Incubate the cells in a humidified incubator with 5% CO2 for 5 min at 37°C.
Add 5 mL of the medium described in the table above and detach cells from the flask by pipetting.
Transfer the cell suspension to a 15 mL tube.
Centrifuge the cells at 800 × g for 3 min at 25°C.
Aspirate the supernatant and resuspend the cell pellet with 5 mL of the medium described in the table above.
Count the cell number by CellDrop.
Note: The cell suspension should be at 0.5 × 106 –2 × 106 cells/mL.
Dilute the cell suspension to 2 × 105 cells/mL with the medium described in the table above.
Seed 10 mL of cell suspension (2 × 105 cells/mL) in a 10 cm dish.
Note: The following cultures should be prepared: one culture for the tested sample and another for the negative control for FACS analysis.
Incubate the cells in a humidified incubator with 5% CO2 overnight at 37°C.
HPG labeling
For the sample, aspirate the medium from one of the 10 cm dishes and add 10 mL of Met-free DMEM (see Recipes).
Aspirate the medium and add 10 mL of Met-free DMEM with anisomycin and HPG (see Recipes) to the 10 cm dish. For the negative control, use DMEM with anisomycin (see Recipes).
Note: Anisomycin is used to shut off cytosolic protein synthesis.
Incubate the 10 cm dish in a humidified incubator with 5% CO2 for 3 h at 37°C.
Fixation and permeabilization
Aspirate the medium from the 10 cm dish and add 10 mL of PBS.
Aspirate the PBS and add 1.5 mL of trypsin-EDTA.
Incubate the cells in a humidified incubator with 5% CO2 for 5 min at 37°C.
Transfer the cell suspension to a 2 mL tube.
Centrifuge at 300 × g for 3 min at 25°C.
Aspirate the supernatant and resuspend the cell pellet with 0.5 mL of cold PBS.
Centrifuge the cells at 300 × g for 3 min at 25°C.
Aspirate the supernatant and resuspend the cell pellet with 0.5 mL of mitochondria-protective buffer with 0.0005% digitonin (see Recipes) at room temperature.
Incubate the cells for 5 min at 25°C.
Centrifuge the cells at 300 × g for 3 min at 25°C.
Discard the supernatant with a pipette and resuspend the cell pellet with 0.5 mL of ice-cold 4% paraformaldehyde phosphate buffer solution on ice.
Incubate the cells for 15 min on ice.
Centrifuge the cells at 300 × g for 3 min at 25°C.
Discard the supernatant with a pipette and resuspend the cell pellet with 0.5 mL of PBS with 0.1% Triton X-100 (see Recipes) at room temperature.
Incubate the cells for 5 min at 25°C.
Centrifuge the cells at 1,000 × g for 3 min at 25°C.
Discard the supernatant and proceed to the “Click reaction” immediately.
Click reaction
Resuspend the cell pellet with 250 µL of click-reaction mixture (see Recipes) at room temperature.
Incubate the cells for 30 min at 25°C.
Centrifuge at 1,000 × g for 3 min at 25°C.
Discard the supernatant with a pipette and proceed to the “Tom20 immunostaining” immediately.
Tom20 immunostaining
Resuspend the cell pellet with 0.5 mL of intercept (TBS) blocking buffer at room temperature.
Centrifuge at 1,000 × g for 3 min at 25°C.
Discard the supernatant with a pipette and resuspend the cell pellet with 100 µL of ice-cold intercept (TBS) blocking buffer with Alexa-Fluor 647 Anti-Tom20 antibody (see Recipes) on ice.
Note: For negative control of Tom20 immunostaining, use ice-cold intercept (TBS) blocking buffer (omitting Alexa-Fluor 647 Anti-Tom20 antibody).
Incubate the cells for 1 h on ice.
Centrifuge at 1,000 × g for 3 min at 2°C.
Discard the supernatant with a pipette and resuspend the cell pellet in 0.5 mL of cold PBS.
Repeat steps E41–42 twice (for a total of three washes).
Resuspend the cell pellet in 0.5 mL of cold FACS buffer (see Recipes).
Proceed to “FACS preparation and analysis” immediately.
FACS preparation and analysis
Filter the cell suspension with a cell strainer and collect the cells in a 5 mL round-bottom polystyrene test tube.
Transfer the cells that passed through the filter to a new 5 mL round-bottom polystyrene test tube and preserve in ice until measurement.
Place the 5 mL round-bottom polystyrene test tube containing the negative control (neither HPG-Cy3 nor Tom20-AF647 labeling) on the BD FACSMelody and start the flow.
Set appropriate photomultiplier tube (PMT) voltages for forward scatters (FSCs) and side scatters (SSCs) with BD FACSChorus so that the signal lies within the detectable range. Then, gate a cell population as “Gate: Cells” in BD FACSChorus.
To discriminate doublets, set “Gate: Singlet” according to the values of FSC-A/FSC-H and FSC-H/FSC-W in FlowJo.
Set the PMT voltages for HPG-Cy3 labeling [561 nm laser, PE filter set (582/15 filter and 582 LP mirror); alternatively, 488 nm laser, PE filter set (586/42 filter and 560 LP)] and Tom20-AF647 labeling [640 nm laser, APC filter set (660/10 filter and 660/10 mirror)] in BD FACSChorus so that the signal is shown in approximately 100.
Replace the 5 mL round-bottom polystyrene test tube containing the sample with the one containing the negative control in BD FACSMelody and start the flow.
Record the data up to 10,000 events in “Gate: Singlet” and then export all the data at least including FSC-A, SSC-A, PE-A, and APC-A as an FCS file.
Data analysis
Load the FCS file in FlowJo.
Readjust the gating to discriminate the dead cells, doublet, and triplet as a new population: “New singlet population.”
Export “New singlet population” as a csv file containing APC-A (Tom20-AF647 signal) and PE-A (HPG-Cy3 signal).
Note: The representative data shown inFigure 1are provided in representative csv files (“HEK293_WithoutHPG,” “HEK293_WithHPG.csv,” “H441#2.csv,” and “H2122#2.csv”) in the Supplemental material .
Load the csv file to R and run “Mito-FUNCAT_FACS_script_Fig1AB.R” or “Mito-FUNCAT_FACS_script_Fig1CD.R” (found in Supplemental items) to conduct the following steps.
Extract 10,000 rows from the top of the csv file.
Remove rows containing fluorescence intensity less than 0.
Normalize the HPG-Cy3 value by the Tom20-AF647 value for each row.
Visualize the distribution of AF647-normalized Cy3 values in the density plot (seeFigure 1for representative data).
Note: To determine statistical significance between samples, we typically used the MannWhitney U test.
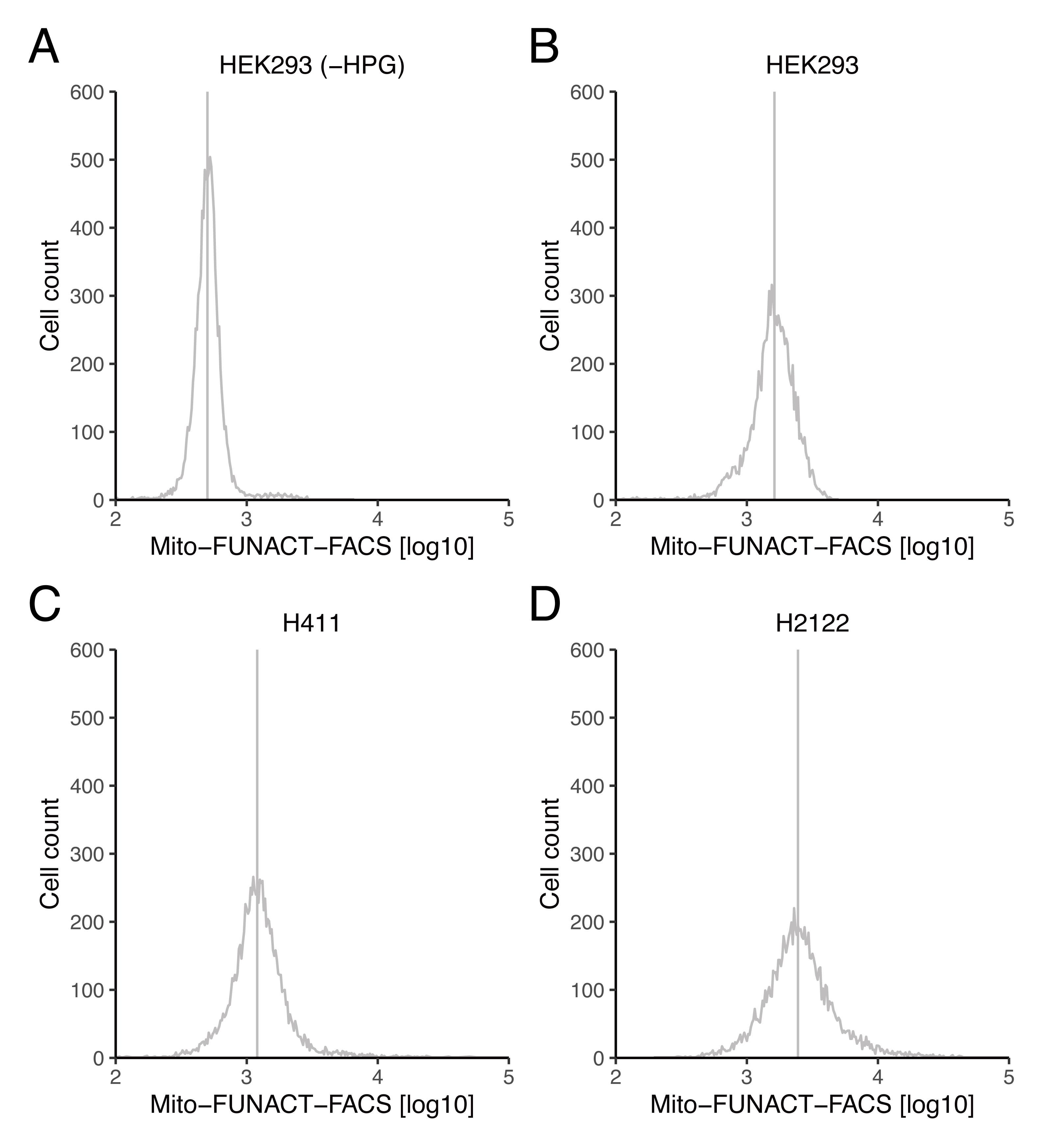
Figure 1. Distribution of Tom20-AF647 (mitochondrial mass)–normalized HPG-Cy3 (mitochondrial translation) signals. (A–D) The Cy3-conjugated nascent peptide in mitochondria was normalized to AF647-labeled Tom20 abundance for HEK293 (A and B), H411 (C), and H2122 (D). For A, HPG was omitted from the medium as the negative control. The vertical lines represent the median of the distributions. The original data (“HEK293_WithoutHPG,” “HEK293_WithHPG.csv,” “H441#2.csv,” and “H2122#2.csv”) and scripts (“Mito-FUNCAT_FACS_script_Fig1AB.R” and “Mito-FUNCAT_FACS_script_Fig1CD.R”) that were used to generate these graphs are provided in the Supplemental material .
Recipes
Met-free DMEM (500 mL)
Reagent Final concentration Amount L-cystine dihydrochloride 48 µg/mL 24 mg 200 mM L-alanyl-L-glutamine solution 4.08 mM 10.2 mL FBS 10% 50 mL DMEM (4.5 g/L D-glucose, no L-glutamine, no sodium pyruvate, no L-methionine and L-cystine) n/a 439.8 mL 100 mg/mL anisomycin (250 µL)
Reagent Final concentration Amount Anisomycin 100 mg/mL 25 mg DMSO n/a 250 µL 100 mM HPG (6.11 mL)
Reagent Final concentration Amount HPG 100 mM 100 mg DMSO n/a 6.11 mL Met-free DMEM with anisomycin and HPG (10 mL)
Reagent Final concentration Amount Met-free DMEM n/a 10 mL 100 mg/mL anisomycin 100 µg/mL 10 µL 100 mM HPG 100 µM 10 µL Note: Prepare before use.
Met-free DMEM with anisomycin (10 mL)
Reagent Final concentration Amount Met-free DMEM n/a 10 mL 100 mg/mL anisomycin 100 µg/mL 10 µL Note: Prepare before use.
0.5% digitonin (10 mL)
Reagent Final concentration Amount Digitonin 0.5% 50 mg RNase-free water n/a 10 mL Mitochondria-protective buffer (5 mL)
Reagent Final concentration Amount Sucrose 10% (w/v) 0.5 g (corresponds to 370 μL) 5 M NaCl 10 mM 10 µL 1 M MgCl2 5 mM 20 µL 1 M HEPES-KOH pH 7.5 10 mM 50 µL RNase-free water n/a 4.55 mL Mitochondria-protective buffer with 0.0005% digitonin (5 mL)
Reagent Final concentration Amount Mitochondria-protective buffer n/a 5 mL 0.5% digitonin 0.0005% 5 µL 10% Triton X-100 (40 mL)
Reagent Final concentration Amount Triton X-100 10% 4 mL RNase-free water n/a 36 mL PBS with 0.1% Triton X-100 (0.5 mL)
Reagent Final concentration Amount PBS n/a 0.5 mL 10% Triton X-100 0.1% 5 µL 5 mM Cy3-azide (370 µL)
Reagent Final concentration Amount Cy3-azide (MW: 539.35) 5 mM 1 mg DMSO n/a 370 µL 50 µM Cy3-azide (100 µL)
Reagent Final concentration Amount 5 mM Cy3-azide 50 µM 1 µL RNase-free water n/a 99 µL Note: Prepare before use.
Click-reaction mixture (1 mL)
Reagent Final concentration Amount 10× Click-iT cell reaction buffer 1× 100 µL 100 mM CuSO4 2 mM 20 µL Click-iT reaction buffer additive n/a 100 µL 50 µM Cy3-azide 1 µM 20 µL RNase-free water n/a 760 µL Note: Prepare before use.
Intercept (TBS) blocking buffer with Alexa-Fluor 647 Anti-Tom20 antibody (101 µL)
Reagent Final concentration Amount Intercept (TBS) blocking buffer n/a 100 µL Alexa-Fluor 647 Anti-Tom20 antibody n/a 1 µL Note: Prepare before use and keep on ice.
FACS buffer
Reagent Final concentration Amount PBS n/a 100 mL Bovine serum albumin 3% 3 g Note: Prepare before use.
Acknowledgments
We are grateful to Yusuke Kimura, Taisei Wakigawa, Yasuhiro Ikegami, Kenji Ohtawa for their constructive advice on the experiments and to all the members of the Iwasaki laboratory for their critical reading of the manuscript. We also thank the Komaba Analysis Core, Institute of Industrial Science, The University of Tokyo, for FACS analysis. This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (JP20H05784 to S.I.; JP20H05786 to Y.I.) and the Japan Agency for Medical Research and Development (AMED) (JP21gm1410001 to S.I. and Y.I.). H.S. was a RIKEN Junior Research Associate (JRA).
This protocol was derived from an original paper (Kimura et al., 2022) and the manufacturer's protocol for the Click-it Cell Reaction Buffer Kit .
Competing interests
The authors declare no competing interests.
Ethics
No human or animal subjects were included in this study.
References
- Anderson, S., Bankier, A. T., Barrell, B. G., de Bruijn, M. H., Coulson, A. R., Drouin, J., Eperon, I. C., Nierlich, D. P., Roe, B. A., Sanger, F., et al. (1981). Sequence and organization of the human mitochondrial genome. Nature 290(5806): 457-465.
- Antonicka, H., Sasarman, F., Nishimura, T., Paupe, V. and Shoubridge, E. A. (2013). The mitochondrial RNA-binding protein GRSF1 localizes to RNA granules and is required for posttranscriptional mitochondrial gene expression. Cell Metab 17(3): 386-398.
- Apostolopoulos, A. and Iwasaki, S. (2022). Into the matrix: current methods for mitochondrial translation studies. J Biochem 171(4): 379-387.
- Chomyn, A. (1996). In vivo labeling and analysis of human mitochondrial translation products. Methods Enzymol 264: 197-211.
- Dieterich, D. C., Hodas, J. J., Gouzer, G., Shadrin, I. Y., Ngo, J. T., Triller, A., Tirrell, D. A. and Schuman, E. M. (2010). In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nat Neurosci 13(7): 897-905.
- Estell, C., Stamatidou, E., El-Messeiry, S. and Hamilton, A. (2017). In situ imaging of mitochondrial translation shows weak correlation with nucleoid DNA intensity and no suppression during mitosis. J Cell Sci 130(24): 4193-4199.
- Fung, S., Nishimura, T., Sasarman, F. and Shoubridge, E. A. (2013). The conserved interaction of C7orf30 with MRPL14 promotes biogenesis of the mitochondrial large ribosomal subunit and mitochondrial translation. Mol Biol Cell 24(3): 184-193.
- Gao, F., Wesolowska, M., Agami, R., Rooijers, K., Loayza-Puch, F., Lawless, C., Lightowlers, R. N. and Chrzanowska-Lightowlers, Z. M. A. (2017). Using mitoribosomal profiling to investigate human mitochondrial translation. Wellcome Open Res 2: 116.
- Gorman, G. S., Chinnery, P. F., DiMauro, S., Hirano, M., Koga, Y., McFarland, R., Suomalainen, A., Thorburn, D. R., Zeviani, M. and Turnbull, D. M. (2016). Mitochondrial diseases. Nat Rev Dis Primers 2: 16080.
- Imami, K., Selbach, M. and Ishihama, Y. (2021). Monitoring mitochondrial translation by pulse SILAC. bioRxiv. https://doi.org/10.1101/2021.01.31.428997
- Iwasaki, S., Floor, S. N. and Ingolia, N. T. (2016). Rocaglates convert DEAD-box protein eIF4A into a sequence-selective translational repressor. Nature 534(7608): 558-561.
- Kashiwagi, K., Shichino, Y., Osaki, T., Sakamoto, A., Nishimoto, M., Takahashi, M., Mito, M., Weber, F., Ikeuchi, Y., Iwasaki, S., et al. (2021). eIF2B-capturing viral protein NSs suppresses the integrated stress response. Nat Commun 12(1): 7102.
- Kimura, Y., Saito, H., Osaki, T., Ikegami, Y., Wakigawa, T., Ikeuchi, Y. and Iwasaki, S. (2022). Mito-FUNCAT-FACS reveals cellular heterogeneity in mitochondrial translation. RNA 28(6): 895-904.
- Li, S. H.-J., Nofal, M., Parsons, L. R., Rabinowitz, J. D. and Gitai, Z. (2021). Monitoring mammalian mitochondrial translation with MitoRiboSeq. Nat Protoc 16(6): 2802-2825.
- Morscher, R. J., Ducker, G. S., Li, S. H.-J., Mayer, J. A., Gitai, Z., Sperl, W. and Rabinowitz, J. D. (2018). Mitochondrial translation requires folate-dependent tRNA methylation. Nature 554(7690): 128-132.
- Pearce, S. F., Rorbach, J., Van Haute, L., D’Souza, A. R., Rebelo-Guiomar, P., Powell, C. A., Brierley, I., Firth, A. E. and Minczuk, M. (2017). Maturation of selected human mitochondrial tRNAs requires deadenylation. Elife 6: e27596.
- Rooijers, K., Loayza-Puch, F., Nijtmans, L. G. and Agami, R. (2013). Ribosome profiling reveals features of normal and disease-associated mitochondrial translation. Nat Commun 4: 2886.
- Sasarman, F. and Shoubridge, E. A. (2012). Radioactive labeling of mitochondrial translation products in cultured cells. Methods Mol Biol 837: 207-217.
- Schöller, E., Marks, J., Marchand, V., Bruckmann, A., Powell, C. A., Reichold, M., Mutti, C. D., Dettmer, K., Feederle, R., Hüttelmaier, S., et al. (2021). Balancing of mitochondrial translation through METTL8-mediated m3C modification of mitochondrial tRNAs. Mol Cell 81(23): 4810-4825.e12.
- Suzuki, T., Yashiro, Y., Kikuchi, I., Ishigami, Y., Saito, H., Matsuzawa, I., Okada, S., Mito, M., Iwasaki, S., Ma, D., et al. (2020). Complete chemical structures of human mitochondrial tRNAs. Nat Commun 11(1): 4269.
- Yousefi, R., Fornasiero, E. F., Cyganek, L., Montoya, J., Jakobs, S., Rizzoli, S. O., Rehling, P. and Pacheu-Grau, D. (2021). Monitoring mitochondrial translation in living cells. EMBO Rep 22(4): e51635.
- Zhang, X., Zuo, X., Yang, B., Li, Z., Xue, Y., Zhou, Y., Huang, J., Zhao, X., Zhou, J., Yan, Y., et al. (2014). MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell 158(3): 607-619.
- Zorkau, M., Albus, C. A., Berlinguer-Palmini, R., Chrzanowska-Lightowlers, Z. M. A. and Lightowlers, R. N. (2021). High-resolution imaging reveals compartmentalization of mitochondrial protein synthesis in cultured human cells. Proc Natl Acad Sci U S A 118(6).
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Saito, H., Osaki, T., Ikeuchi, Y. and Iwasaki, S. (2023). High-throughput Assessment of Mitochondrial Protein Synthesis in Mammalian Cells Using Mito-FUNCAT FACS. Bio-protocol 13(3): e4602. DOI: 10.21769/BioProtoc.4602.
Category
Molecular Biology > Protein > Flow Cytometry
Cell Biology > Cell staining > Protein
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









