- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation and Characterization of DNA-assembled GRS-DNA-CuS Nanodandelions
(*contributed equally to this work) Published: Vol 13, Iss 2, Jan 20, 2023 DOI: 10.21769/BioProtoc.4595 Views: 1718
Reviewed by: ASWAD KHADILKARValeria B Fernandez ValloneAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Preparation and Characterization of Ginger Lipid-derived Nanoparticles for Colon-targeted siRNA Delivery
Junsik Sung [...] Didier Merlin
Jul 20, 2020 4804 Views
Preparation and Characterization of Lipid Nanoparticles Co-loaded With DNA and Nitro-Oleic Acid
Manthan N. Patel [...] Jacob S. Brenner
Sep 20, 2025 3135 Views
Cost-Effective and Reproducible Preparation of mRNA-Loaded Lipid Nanoparticles Using a Conventional Laboratory-Scale Microfluidic Assembly System
Yunqi Li [...] Ruoyang Zhao
Sep 20, 2025 1431 Views
Abstract
In this study, we introduce a detailed protocol for the preparation of DNA-assembled GRS-DNA-copper sulfide (CuS) nanodandelion, a multifunctional theranostics nanoparticle. Using transmission electron microscope (TEM) and dynamic light scattering techniques, we characterize the physicochemical property of DNA-assembled GRS-DNA-CuS nanodandelions and their dissociation property after the first near-infrared (NIR) light irradiation. In addition, we systematically monitor the processes of tumor accumulation and uniform intratumoral distribution (UITD) of ultrasmall CuS photothermal agents (PAs), which are dissociated from GRS-DNA-CuS nanodandelions, by Raman imaging and photoacoustic imaging, respectively. The UITD of the dissociated ultrasmall CuS PAs can enhance the therapeutic efficiency of photothermal treatment under the second NIR light irradiation. Overall, this protocol provides a powerful tool to achieve UITD of PAs by explosively breaking the hydrogen bonds of DNA in GRS-DNA-CuS nanodandelions under NIR light irradiation. We expect DNA-assembled nanotheranostics to serve as a robust platform for a variety of biomedical applications related to photothermal therapy in the oncology field. This protocol can increase experimental reproducibility and contribute to efficient theranostics nanomedicine.
Keywords: DNA assemblyBackground
Although traditional cancer treatments (e.g., chemotherapy, radiotherapy, and surgery) have been widely applied in clinical cancer therapy, they have many side effects (such as systemic toxicity or incomplete elimination), leading to poor therapeutic efficiency and easy recurrence and metastasis. In recent years, photothermal therapy (PTT) has been broadly investigated because of its advantages in deeper tissue penetration, higher spatial selectivity, and local tumor thermal ablation with less systemic toxicity and higher anti-tumor efficiency (Xiong et al., 2020; Zhou et al., 2021). However, due to the non-uniform tumor distribution of photothermal agents (PAs), PTT cannot kill all tumor cells (Yan et al., 2016). Therefore, it is urgent to develop a novel method to achieve uniform intra-tumor distribution (UITD) of PAs and high photothermal therapeutic efficiency.
Several size shrinkage strategies based on cross-linking covalent bonds have been developed to improve UITD of PAs (Li et al., 2016; Zhou et al., 2019; Wang et al., 2020). However, covalent bonds with high bond energy cannot be broken quickly and the synthesis of size-shrinkable nanosystems is complex, which easily results in differences in physicochemical characteristics between different batches. Because of the low hydrogen bond energy in the DNA double helix and the simplicity of DNA self-assembling process, DNA-assembled nanotheranostics may be a promising platform to achieve UITD of PAs.
In this protocol, we will introduce a DNA-assembled nanosystem, i.e., DNA-assembled GRS-DNA-CuS nanodandelion (as shown in Figure 1), which is composed of the core of a Gap-enhanced rod-shaped gold structure (termed as GRS) modified by single-stranded DNA (ssDNA), and the shell of ultrasmall copper sulfide (CuS) PAs modified by complementary single-stranded DNA (cssDNA) (Zhang et al., 2022). Based on the principle of Watson–Crick base pairing, GRS-DNA-CuS nanodandelions are prepared by cross-linking hydrogen bonds between GRS-ssDNA and CuS-cssDNA PAs. Under the first near-infrared (NIR) laser irradiation, hydrogen bonds in the GRS-DNA-CuS nanodandelions are explosively broken once above the DNA melting temperature, and the large-sized GRS-DNA-CuS nanodandelions are dissociated into GRS and ultrasmall CuS PAs within one minute (Figure 1). Both the rapid increase in local concentration of ultrasmall CuS PAs and the slight increase in intratumor temperature have effectively promoted UITD of CuS PAs. This UITD helps to eliminate the subcutaneous solid tumors under the second NIR light irradiation. In a word, we will present the detailed preparation process of DNA-assembled GRS-DNA-CuS nanodandelions and characterize their physicochemical and dissociation properties in order to provide a novel UITD-guided PTT strategy and advance their therapeutic and diagnostic applications in clinic.
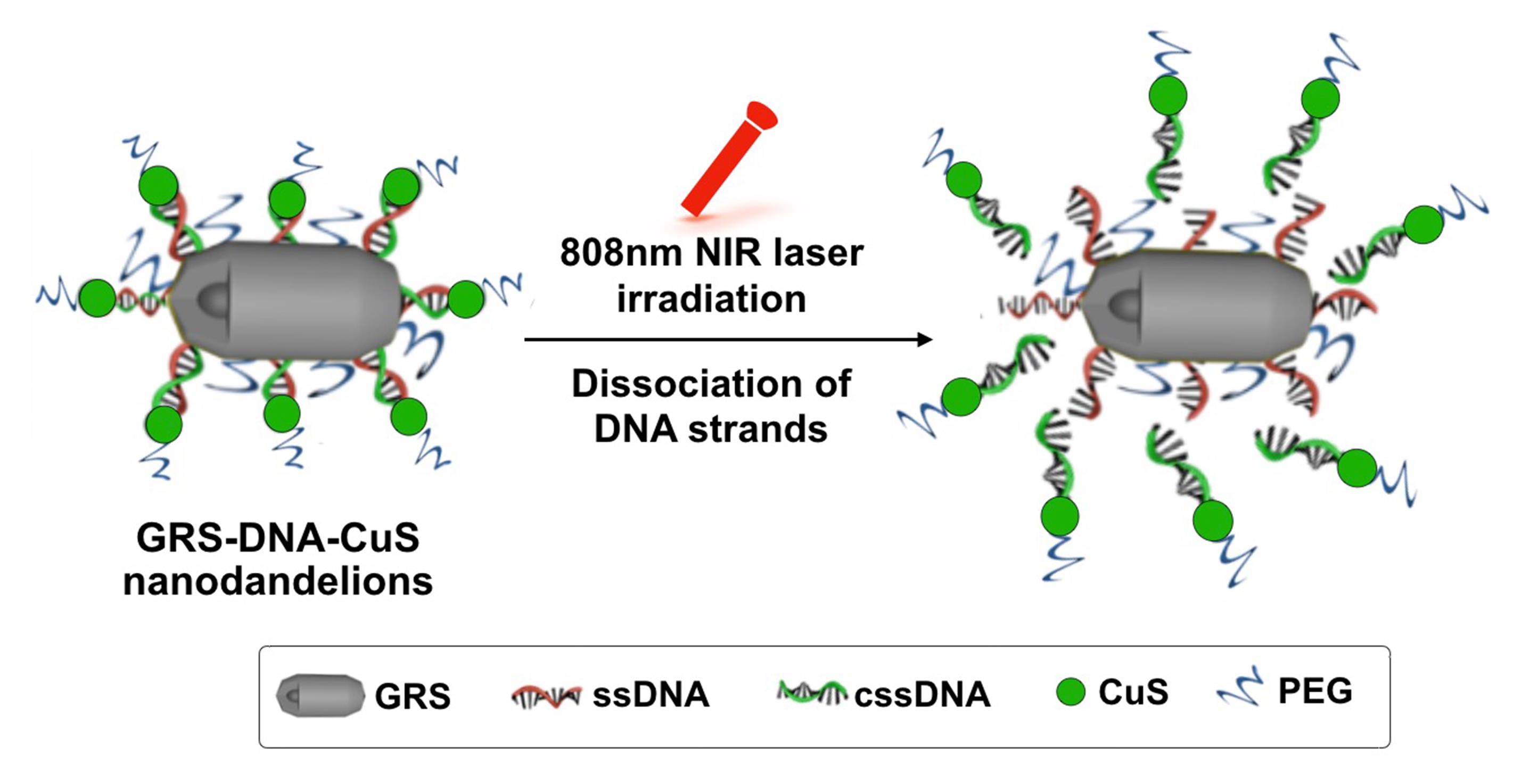
Figure 1. Schematic illustration of the dissociation process of DNA-assembled GRS-DNA-CuS nanodandelions under 808 nm NIR laser irradiation. Left: 3D structure of DNA-assembled GRS-DNA-CuS nanodandelions. GRS: Gap-enhanced rod-shaped gold structure; CuS: copper sulfide.
Materials and Reagents
15 mL centrifuge tube (BIOFIL, catalog number: CFT013150-BD)
50 mL centrifuge tube (BIOFIL, catalog number: CFT010500)
Gold (III) chloride hydrate (HAuCl4·4H2O) (Sinopharm Chemical Reagent Co., Ltd, catalog number: 10010711)
Cetyl trimethyl ammonium bromide (CTAB) (Aladdin, catalog number: H108983-500g)
Sodium borohydride (NaBH4) (Aladdin, catalog number: S108355-25g)
Sodium oleate (NaOL) (Aladdin, catalog number: S104196-250g)
Silver nitrate (AgNO3) (Aladdin, catalog number: S116268)
Hydrogen chloride (HCl) (Merck, Sigma-Aldrich, catalog number: 258148)
Ascorbic acid (AA) (Aladdin, catalog number: 50-81-7)
4-Nitrobenzenethiol (4-NBT) (Aladdin, catalog number: N298977)
Cetanecyl trimethyl ammonium chloride (CTAC) (Aladdin, catalog number: H105309)
mPEG5k -SH (JenKem Technology, catalog number: 729108-1G)
Tween 20 (Aladdin, catalog number: T104863-100ml)
Sodium citrate dihydrate (Aladdin, catalog number: S434901)
Cupric chloride (CuCl2) (Aladdin, catalog number: C106774-50g)
Single-stranded DNA (ssDNA, sequence: 5′-TAG GTG TAG TGA GGA AAA AAA AAA A-C6-SH-3′, 200 μL, 10 μM) (Sangon Biotech Limited Co. Shanghai, China, personalized customization)
Complementary single-stranded DNA (cssDNA, sequence: 5′-TCC TCA CTA CAC CTA AAA AAA AAA A-C6-SH-3′, 380 μL, 10 μM) (Sangon Biotech Limited Co. Shanghai, China, personalized customization)
Vernier caliper (Shanghai Shenhan Measures, catalog number: 43528447520)
Pipettes (Biosharp, catalog number: BS-XG-0.3L)
Capillary cell (MALVERN, catalog number: DTS1070)
PBS (Sangon Biotech, catalog number: B640435)
Balb/c nude mice (Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China))
Equipment
Centrifuge (Thermo Fisher, model: Heraeus Fresco 21)
-80°C freezer (Thermo Fisher, model: ORMA 88000 SERIES)
Water purification system (Millipore, model: Direct-Q5)
Hot plate magnetic stirrer (IKA, model: C-MAG HS7 digital)
Electron microscopy (FEI, model: FEI-TEM)
Malvern laser particle size analyzer (Malvern 3000, Zetasizer Nano ZS)
Inductively coupled plasma–mass spectrometry, ICP-MS (VARIAN, model: 710-ES)
Renishaw inVia confocal Raman spectrometer (Renishaw, model: inVia)
Ultracentrifuge (Eppendorf, model: Centrifuge 5804R)
Ultrasonic cell disruptor system (SONICS, model: S-450D)
Rapid mixer (Kylin-Bell, model: Vortex-5)
Ultrasonic cleaner (Scientz, model: SB-5200DTS)
Thermal imaging camera(FLIR, model: AX5)
Ultracentrifuge (Optima, model: XPN-80)
Procedure
Preparation of DNA-assembled GRS-DNA-CuS nanodandelions
Synthesis of gold nano-rods (GRs)Mix 5 mL of HAuCl4·4H2O (0.2 mg/mL) with 5 mL of CTAB (0.2 M).
Dilute 20 μL of fresh NaBH4 (0.6 M) in 1 mL of ice-cold water and add it to the mixture under vigorous stirring at 1793 × g for 1 min. Then, the seed solution CTAB-stabilized gold core is obtained.
Add 0.9 g of CTAB and 0.15 g of NaOL into 25 mL of double-distilled water (ddH2O) at 50°C under magnetic stirring for 1 h.
Note: ddH2O should be warmed to 50°C in advance.
Cool the solution from the previous step to 30°C and add 2.4 mL of AgNO3 (4 mM) and 25 mL of HAuCl4·4H2O (0.4 mg/mL). Let them stand at 30°C for 10 min and stir again for 15 min at 747 × g .
Note: Add the ice to the water bath in order to cool down the hot water. Real-time monitor the temperature of water bath by thermometer so that the reaction temperature is kept at 30°C.
Add 0.24 mL of HCl (37 weight percent) and stir for another 15 min at 747 × g . Then, add 125 μL of AA (0.064 M) and stir for 1 min to obtain the growth solution .
Let the obtained growth solution stand for 12 h at 30°C for GR growth. Collect the resulting products by centrifugation at 9,710 × g for 20 min, wash with 40 mL of ddH2O, re-dissolve into 50 mL of ddH2O, and obtain gold nano-rods (GRs).
Note: Rotating speed should be 9,710 × g in order to totally collect GRs.
Finally, mix 5 mL of GRs solution with 5 mL of CTAC (0.2 M) and centrifuge at 11,203 × g for 10 min. Wash the precipitate with 10 mL of CTAC (0.1 M) three times and re-disperse them in 10 mL of CTAC solution (0.1 M) for completely replacing CTAB. CTAC-capped GRs are obtained.
Note: Both the long sonication time and high solution temperature are helpful to completely dissolve CTAC and CTAB in the ddH2O. The better temperature for dissolving them is 45°C. Optimal sonication time is 30 min. In addition, during the displacement of CTAB with CTAC, the samples should be dispersed by sonication before proceeding to the next step, as they easily adsorb on the surface of tube.
Mix 10 mL of CTAC-capped GRs with 500 μL of Raman molecule 4-NBT (1 mM) under vigorous ultrasonication in an ice-water bath in an ultrasonic cleaner for 15 min.
Note: Raman molecule 4-NBT should be dissolved in DMSO in advance. The concentration of 4-NBT is 1 mM.
Add 10 mL of CTAC (0.1 M) to the solution and centrifuge at 8,963 × g for 10 min. Wash the precipitate with 10 mL of CTAC solution (0.05 M) twice and re-disperse the precipitate in 10 mL of CTAC solution (0.1M). The precipitate is Raman molecule-modified GR .
Sequentially add 2.5 mL of HAuCl4·4H2O (2 mg/mL) and 2.5 mL of AA (0.04 M) to 50 mL of CTAC (0.1 M).
Add the mixture to 5 mL of Raman molecule-modified GR solution drop by drop under continuous sonication.
Let the mixture solution stand at room temperature for 24 h, centrifuge at 4,481 × g for 10 min, and re-disperse them in 50 mL of ddH2O. Then, GRS is obtained.
Centrifuge the obtained GRS (20 mL) at 7469 × g for 10 min and wash twice with ddH2O. Then, add 200 μL of mPEG5k-SH (10 μM) to the GRS solution.
Subsequently, add 20 mL Tween 20 (0.01 weight percent) to PEG-modified GRS solution, sonicate for 30 s, and severely mix by vortex. Repeat this step four times.
Note: The processes including adding Tween 20, sonicating, and severely mixing by vortex should be repeated four times in order to completely remove the CTAC from the surface of PEG-modified GRS. Zeta potential of PEG-modified GRS should be changed from positive to negative.
Add 200 μL of single-stranded DNA (ssDNA, sequence: 5′-TAG GTG TAG TGA GGA AAA AAA AAA A-C6-SH-3′, 10 μM) and 2 mL of citrate sodium dihydrate (1 M) to the obtained GRS solution, and then mix them for 30 s by vortex. Let the solution stand at room temperature for 2 h.
Note: 2.894 g of citrate sodium dihydrate is dissolved in 10 mL of ddH2O to obtain citrate solution (1 M).
Centrifuge the ssDNA-modified GRS, wash three times with ddH2O, and re-dissolve in 20 mL of 1× PBS. GRS-ssDNA is obtained.
Add 13.45 mg of CuCl2 and 20 mg of citrate sodium dihydrate to 100 mL of ddH2O.
Add 8.68 mg of Na2S into the mixture from the previous step under stirring. Five minutes later, heat the reaction solution to 90 and stir for 15 min.
Keep the citrate-modified CuS nanoparticles in ice-cold water for 30 min and store at 4 for less than one week.
Note: Ultrasmall CuS nanoparticles should be prepared prior to use. The storage time of CuS at 4 should be no more than one week because CuS is easily degraded.
Add 380 μL of the complementary single-stranded DNA (cssDNA, 5′-TCC TCA CTA CAC CTA AAA AAA AAA A-C6-SH-3′, 10 μM) to 50 mL of CuS solution and gently shake at room temperature at the incubator shaker at 60 rpm for 12 h.
Centrifuge the mixture at 232,580 × g for 30 min and re-disperse in 50 mL of 1× PBS. CuS-cssDNA is obtained.
Mix 20 mL of GRS-ssDNA with 10 mL of CuS-cssDNA and incubate at 37 for 12 h to generate GRS-DNA-CuS nanodandelions.
Collect the GRS-DNA-CuS nanodandelions by centrifugation in 50 mL centrifuge tubes at 8,963 × g for 10 min and re-dissolve in 20 mL of 1× PBS.
Add 200 μL of mPEG5k-SH (10 μM) to GRS-DNA-CuS nanodandelions for improvement of their stability.
Wash the PEG-modified GRS-DNA-CuS with 20 mL of 1× PBS and re-dissolve in 20 mL of 1× PBS. The whole procedure for preparation of GRS-DNA-CuS nanodandelions is illustrated in Figure 2 .
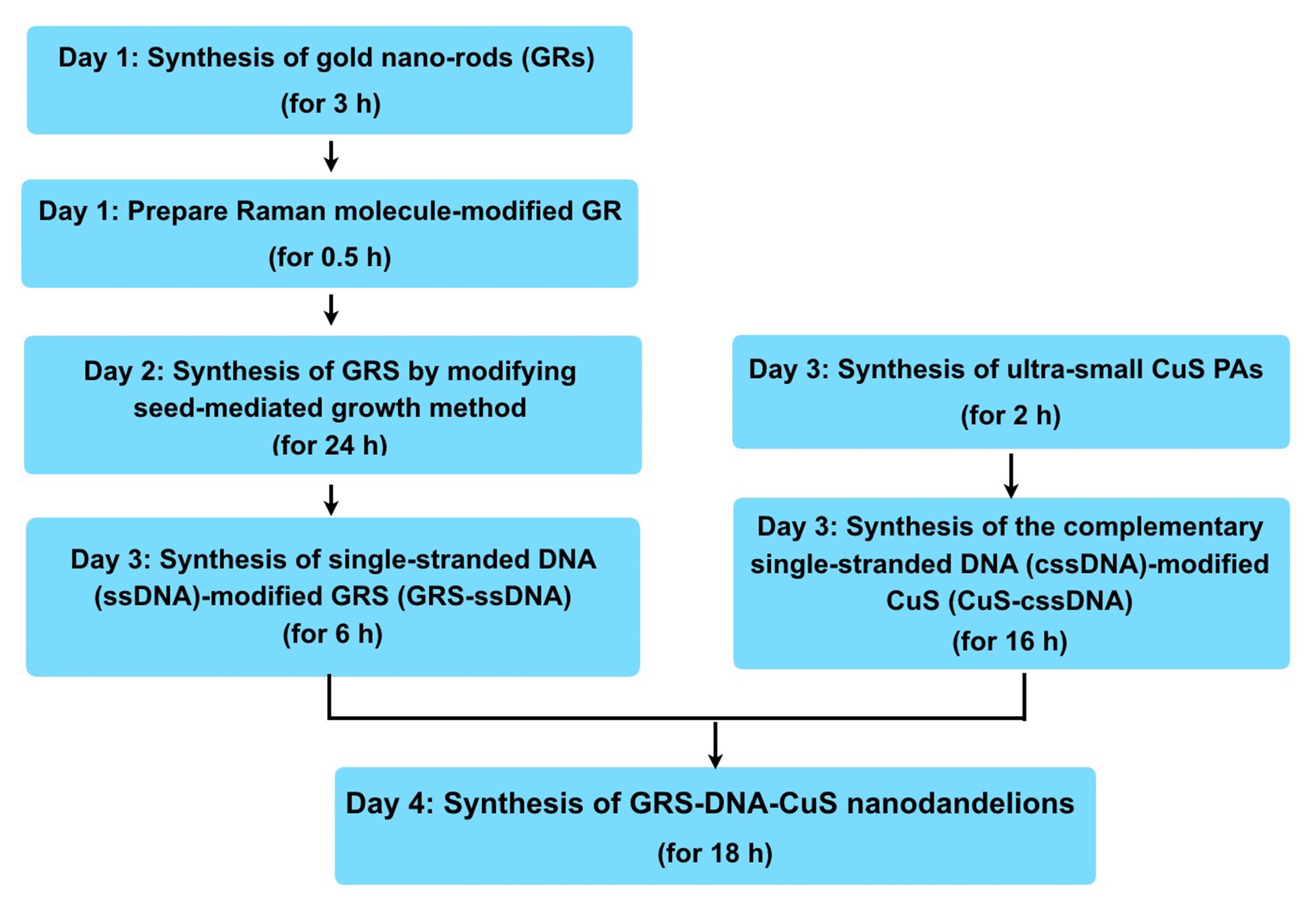
Figure 2. Flow chart of the whole procedure for preparation of GRS-DNA-CuS nanodandelions
Characterization of DNA-assembled GRS-DNA-CuS nanodandelions
Characterize the morphologyDribble 10 µL of the obtained GRS-DNA-CuS solution onto the formvar-coated grid.
Dry the sample at room temperature for 30 min.
Monitor the morphology of GRS-DNA-CuS by transmission electron microscopy (TEM).
Dilute 5 μL of the obtained GRS-DNA-CuS into 1.5 mL of ddH2O.
Add the sample to the cuvette without any bubbles.
Insert the cuvette into the particle analyzer chamber.
Detect the size of GRS-DNA-CuS using the Malvern laser particle size analyzer.
Dilute 5 μL of GRS-DNA-CuS nanodandelions into 1.5 mL of ddH2O.
Add the sample to a capillary cell.
Cover the capillary cell with a plastic cap.
Place the capillary cell into the chamber of the zeta potential analyzer.
Measure the zeta potential value of GRS-DNA-CuS (-32.2 mV) using the Malvern laser particle size analyzer.
Dilute 5 μL of GRS-DNA-CuS nanodandelions in 150 μL of ddH2O.
Drop 20 μL of the diluted GRS-DNA-CuS solution onto the foil-wrapped slide.
Place slide in the instrument and measure Raman signal intensity of GRS-DNA-CuS by Renishaw inVia Raman microscope in StreamLine mode (5× objective, 62.6 mW) with 5 s of exposure time under 830 nm laser excitation, as shown in Figure 3 .

Figure 3. Process for measuring Raman signal intensity of GRS-DNA-CuS nanodandelions
Irradiate GRS-DNA-CuS nanodandelions and CuS nanoparticles (NPs) by 808 nm laser (2.8 W/cm2) for 4 min.
Use a FLIR Ax5 camera to record the temperature changes in each group at an interval of 30 s during the laser irradiation.
Intravenously inject GRS-DNA-CuS nanodandelions at the dose of 50 μmol/kg body weight into A549 tumor-bearing Balb/c male nude mice (approximately 20 g, 6 weeks old).
Note: A549 cells (5 × 106 cells/mouse in 100 μL PBS) are subcutaneously injected into the back of Balb/c nude mice and allowed to grow to the tumor size of 125 mm3 for the imaging and therapeutic studies.
Collect Raman signals of GRS-DNA-CuS nanodandelions at the tumor tissues at 0, 1, 2, 4, 8, 12, and 24 h post-injection, by Renishaw inVia Raman microscope in StreamLine mode (5× objective, 62.6 mW) with 5 s of exposure time under 830 nm laser excitation.
Intravenously inject GRS-DNA-CuS nanodandelions (50 μmol/kg body weight) into A549 tumor-bearing Balb/c male nude mice (approximately 20 g, 6 weeks old).
Irradiate the A549-tumors on Balb/c nude mice for 2 min at 2.8 W/cm2 under 808 nm laser at 12 h post-injection.
Image the photoacoustic signals at the tumor tissues by photoacoustic imaging system at 0, 0.5, 6, and 12 h of post-laser irradiation
Note: In the control group, tumors on mice are intratumorally infected with RS-DNA-CuS nanodandelions without NIR laser irradiation.
Evaluate the photothermal therapeutic efficiency of GRS-DNA-CuS nanodandelionsIntravenously inject GRS-DNA-CuS nanodandelions (50 μmol/kg body weight) into A549 tumor-bearing Balb/c male nude mice (approximately 20 g, 6 weeks old).
Irradiate the tumors at 12 h after injection of GRS-DNA-CuS nanodandelions by the first 808 nm NIR laser.
Note: This first 808 nm NIR laser irradiation is for the dissociation of GRS-DNA-CuS nanodandelions that are accumulated at the tumors.
Six hours later, tumors are irradiated again by the second 808 nm NIR laser.
Note: This second 808 nm NIR laser irradiation is used for the tumor photothermal treatment.
Measure the tumor size by vernier caliper and calculate according to the equation: V = L × W2/2, where the L and W are the tumor size at the longest and widest point, respectively.
Data analysis
Characterize the physiochemical properties (such as morphology, size, zeta potential, Raman signal, and photothermal conversion properties, etc.) of DNA-assembled GRS-DNA-CuS nanodandelions. TEM images in Figure 4 show that the numerous CuS PAs are clearly coated on the surface of GRS, indicating that GRS-DNA-CuS is successfully prepared. Scanning transmission electron microscopy-energy dispersive X-ray spectrometry (STEM-EDX ) elemental images in Figure 5 also indicate the successful formation of GRS-DNA-CuS. The size of GRS-DNA-CuS is approximately 135 nm in Figure 6 . GRS-DNA-CuS exhibits the highest Raman signal intensity at 1331 cm−1, which is beneficial for detecting their intratumoral distribution by Raman imaging technique. The photothermal conversion efficiency of GRS-DNA-CuS is approximately 47.44%, indicating a better photothermal property. Meanwhile, temperature of GRS-DNA-CuS solution increases rapidly from room temperature to 46.8 within 1 min under 808 nm near-infrared irradiation with a laser power density of 2.8 W/cm2 , suggesting that the generated heat is sufficient to induce the explosive breakage of hydrogen bonds of DNA (Tm = 42.82°C) in GRS-DNA-CuS nanodandelions.
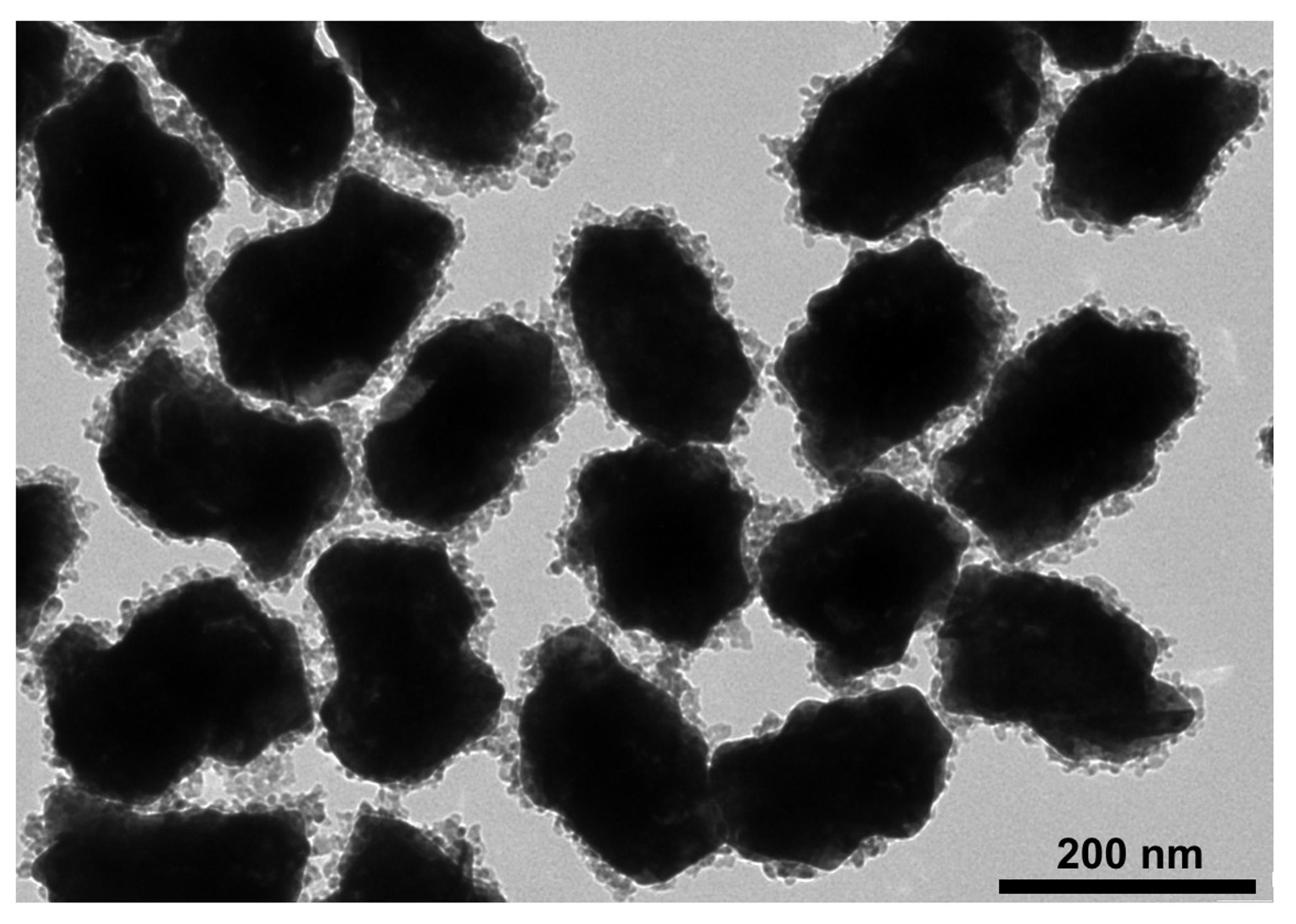
Figure 4. Representative TEM image of GRS-DNA-CuS nanodandelions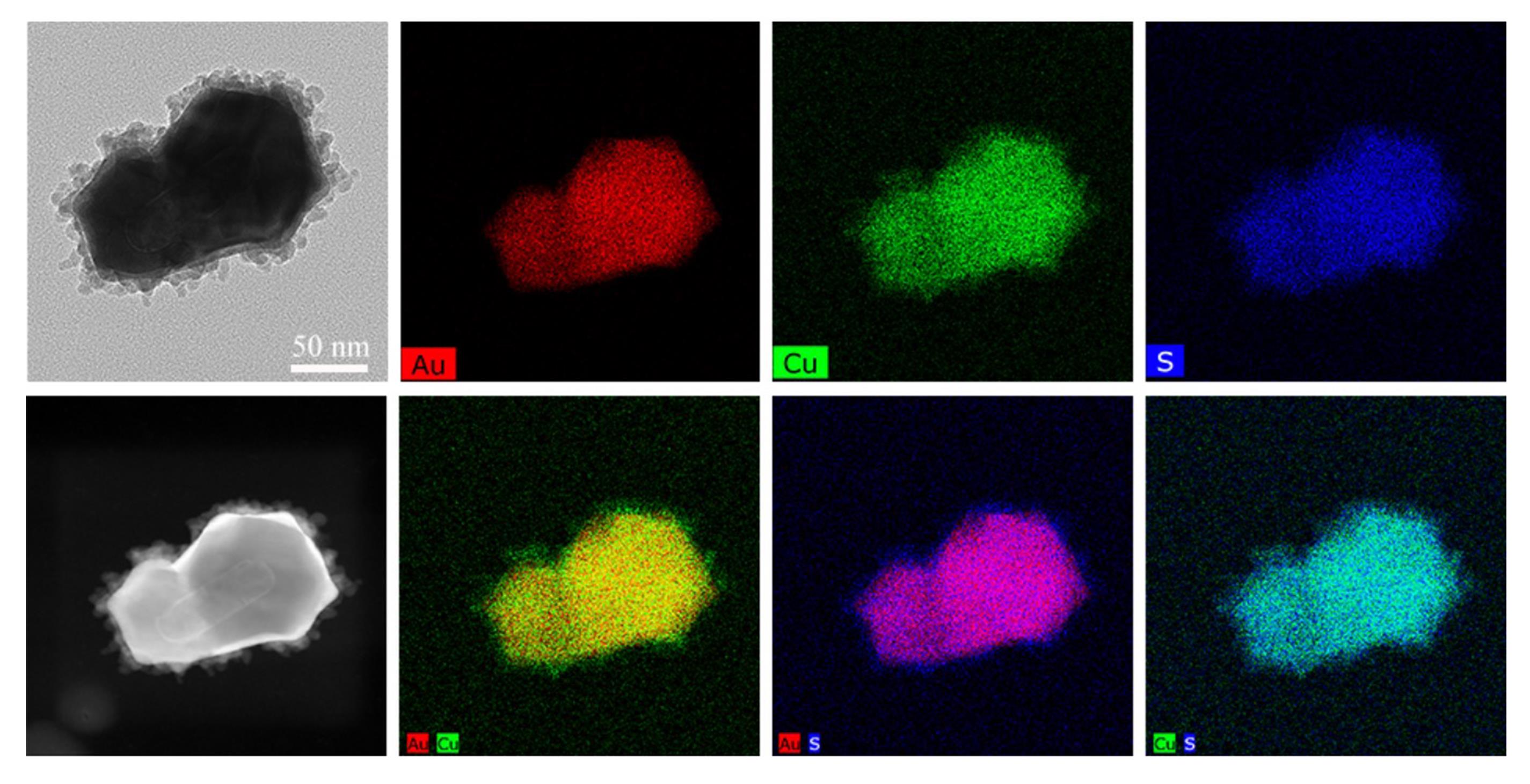
Figure 5. STEM-EDX elemental mapping images of GRS-DNA-CuS nanodandelions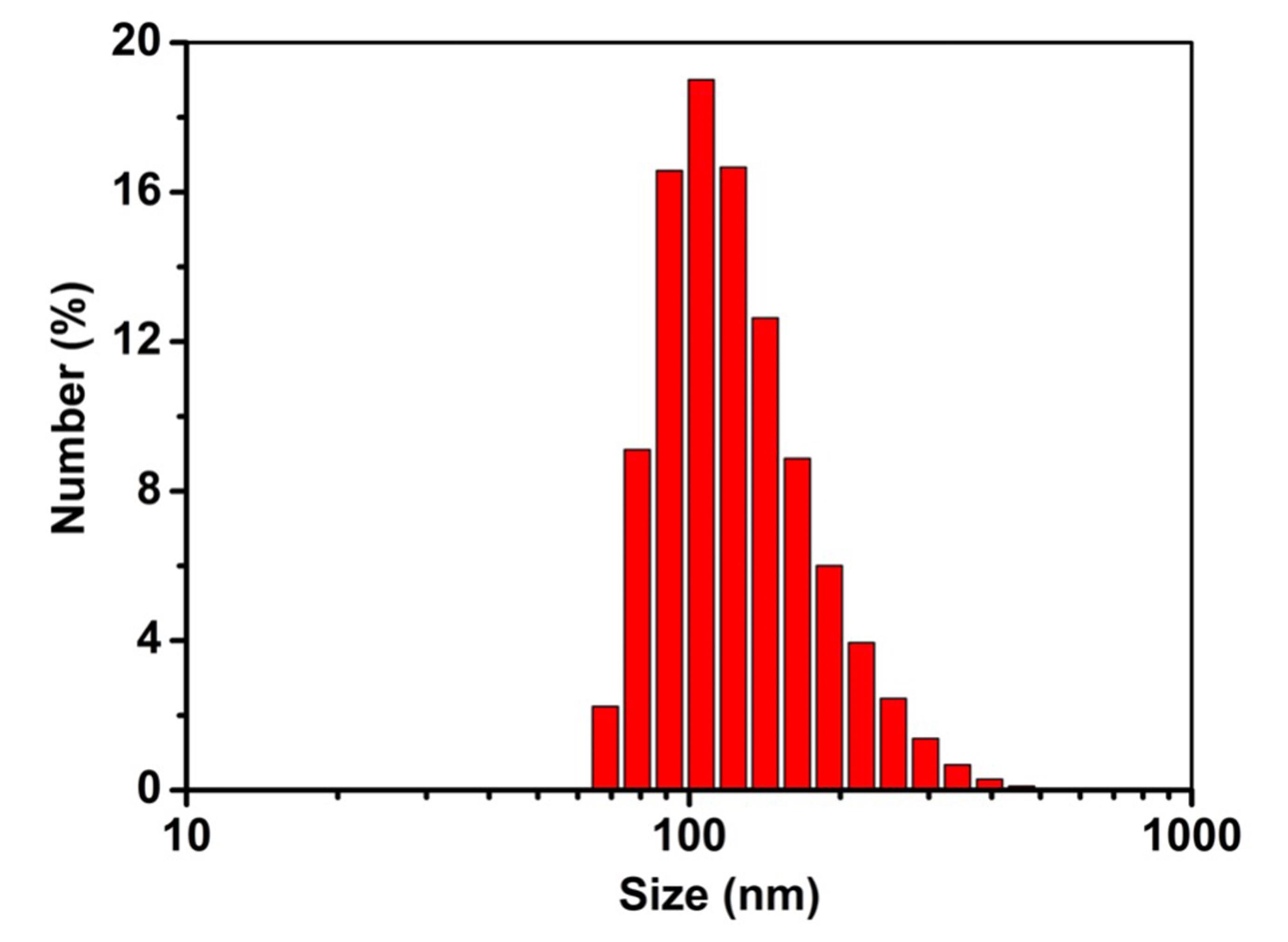
Figure 6. Size distribution of GRS-DNA-CuS nanodandelionsDetect the dissociation of DNA-assembled GRS-DNA-CuS nanodandelions after NIR light irradiation. TEM images in Figure 7 show a complete dissociation of large-sized GRS-DNA-CuS into GRS-ssDNA and ultrasmall CuS-cssDNA PAs within 1 min after 808 nm NIR laser irradiation with a laser power density of 2.8 W/cm2.
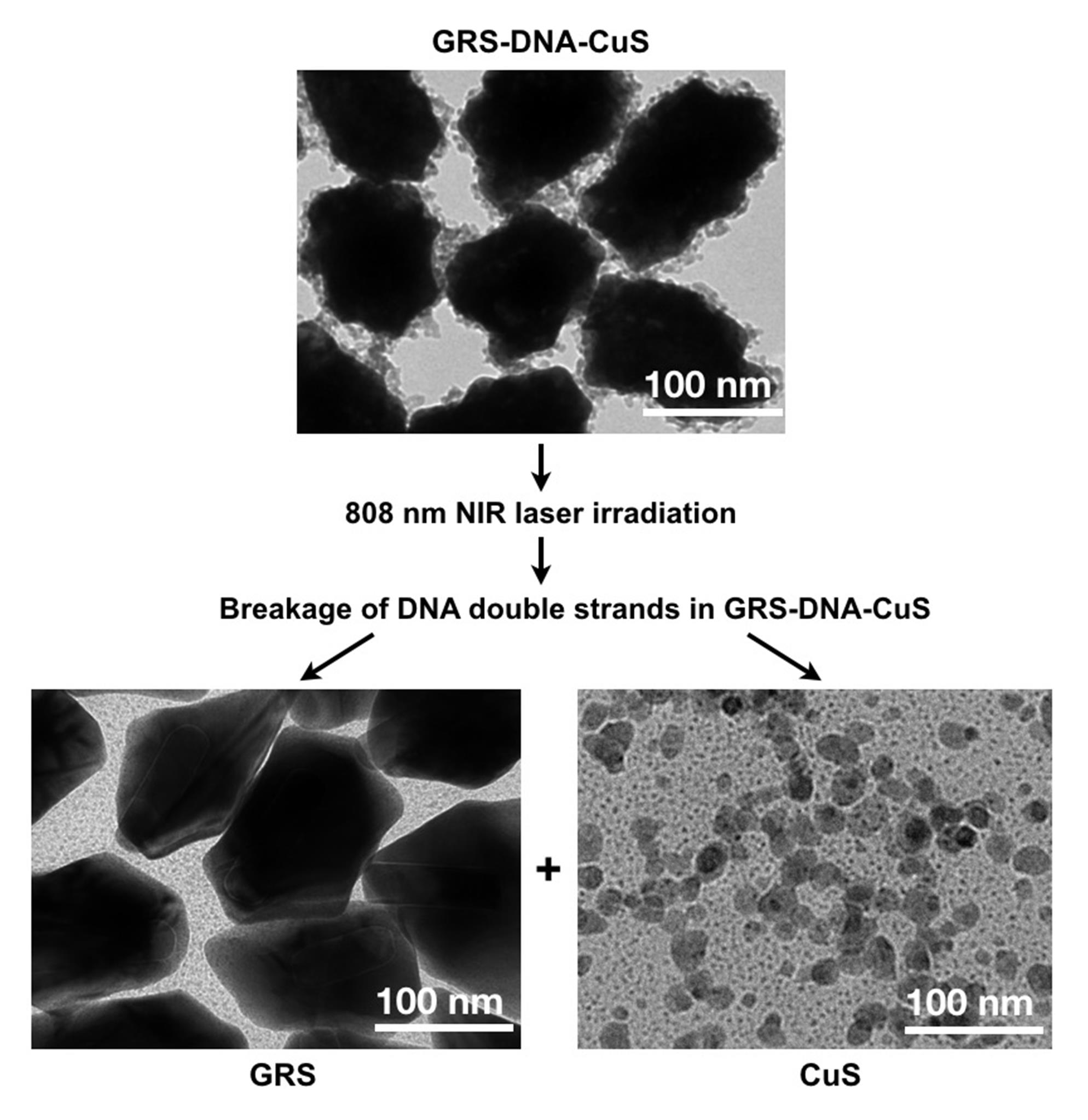
Figure 7. TEM images for detecting morphological changes of GRS-DNA-CuS before and after dissociation under 808 nm NIR laser irradiationMonitor the tumor accumulation process of DNA-assembled GRS-DNA-CuS nanodandelions by Raman imaging technique (as shown in Figure 4A–C in Zhang et al., 2022). GRS-DNA-CuS nanodandelions reach maximum Raman intensity in tumor tissues after 12 h and then remain unchanged until 24 h, demonstrating that NIR-triggered hydrogen-bond thermal breakage in large-sized GRS-DNA-CuS nanodandelions can be conducted between 12 and 24 h after intravenous injection.
Analyze the intra-tumor diffusion process of the ultrasmall CuS PAs dissociated from GRS-DNA-CuS nanodandelions by photoacoustic (PA) imaging. At 12 h post-injection of GRS-DNA-CuS, mice in the NIR-irradiated group are irradiated by NIR laser with a power of 2.8 W/cm2 for 2 min. Accumulated GRS-DNA-CuS nanodandelions at the tumor are entirely dissociated into ultrasmall CuS PA under the first NIR laser irradiation. Higher PA red-positive signals are observed near the tumor center after 6 h of irradiation (Figure 8), indicating that NIR-triggered dissociation contributes to better tumor dispersibility of ultrasmall CuS PAs. The diffusion depth of ultrasmall CuS PAs is greater at 6 h than at 0.5 h after laser irradiation (Figure 8), demonstrating that ultrasmall CuS PAs penetrate at the tumor tissues in a time-dependent manner.
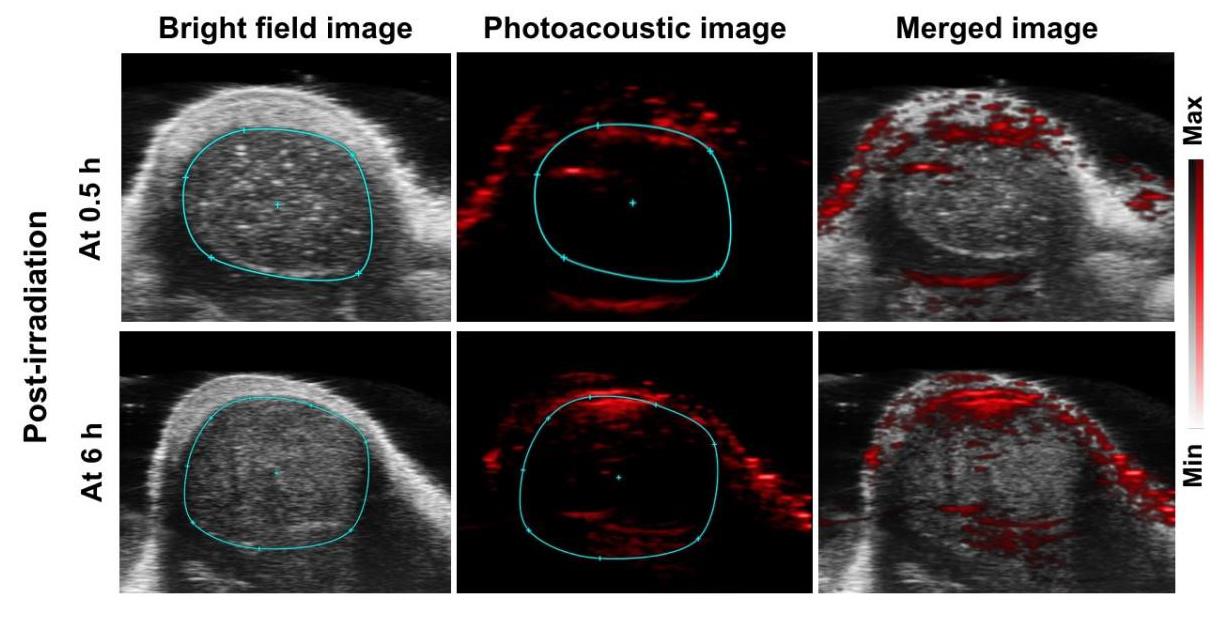
Figure 8. Photoacoustic images of GRS-DNA-CuS nanodandelions at tumor tissues after 0.5 and 6 h post-irradiation.Evaluate UITD-guided PTT efficiency. In the GRS-DNA-CuS-treated group with two NIR laser irradiations, three-fifths of the tumors are completely eliminated and no longer recurred, indicating the role of UITD in PTT-treated tumors (Figure 5B and 5C in Zhang et al., 2022).
Meanwhile, the above physicochemical characterizations and the obtained main results about GRS-DNA-CuS nanodandelions are also illustrated in Table 1.
Table 1. Physicochemical characterizations and the obtained main results of DNA-assembled GRS-DNA-CuS nanodandelions
| Characterization content | Equipment | Obtained main results | |
|---|---|---|---|
| 1 | Morphology of GRS-DNA-CuS | Transmission electron microscopy (TEM) | GRS-DNA-CuS has spherical structure |
| 2 | Size of GRS-DNA-CuS | Malvern laser particle size analyzer | Hydrodynamic size of GRS-DNA-CuS is 135 nm |
| 3 | Zeta potential value of GRS-DNA-CuS | Malvern laser particle size analyzer | Zeta potential of GRS-DNA-CuS is -32.2 mV |
| 4 | Raman signal intensity of GRS-DNA-CuS | Renishaw inVia Raman microscope | GRS-DNA-CuS has the highest Raman signal intensity at 1331 cm−1 |
| 5 | Photothermal effect of GRS-DNA-CuS | 808 nm laser and FLIR Ax5 camera | GRS-DNA-CuS exhibits photothermal conversion efficiency of 47.44% and higher photothermal stability |
| 6 | Intratumoral accumulation | Renishaw inVia Raman microscope | The accumulation amount of GRS-DNA-CuS at tumors can reach the maximum after 12 h of intravenous injection |
| 7 | Intratumoral penetration | Photoacoustic imaging system | GRS-DNA-CuS distributes around the whole tumor after 6 h of irradiation |
| 8 | Photothermal therapeutic efficiency | Vernier caliper | In the treated group with two laser irradiations, three-fifths of the tumors are completely eliminated and no longer recurred |
Acknowledgments
This protocol is based on our previous publication (Zhang et al., 2022). We are supported by National Key Research and Development Program of China (NO. 2020YFA0210800), National Natural Science Foundation of China (NO. 21874092, 52161160307, and 31671003), Startup Fund for Young Faculty at Shanghai Jiao Tong University (NO. 21X010501069), and Shanghai Frontiers Science Center of Cellular Homeostasis and Human Diseases. We also thank the Core Facility of Basic Medical Sciences, Shanghai Jiao Tong University School of Medicine for assistance with in vitro experiments.
Competing interests
The authors declare no conflicts of interest.
Ethics
All the animal procedures were approved by Shanghai Jiao Tong University School of Medicine Ethics Committee and carried out in the experimental animal Center of Shanghai Jiao Tong University School of Medicine.
References
- Zhou, C., Zhang, L., Sun, T., Zhang, Y., Liu, Y., Gong, M., Xu, Z., Du, M., Liu, Y., Liu, G. and Zhang, D. (2021). Activatable NIR-II Plasmonic Nanotheranostics for Efficient Photoacoustic Imaging and Photothermal Cancer Therapy. Adv Mater 33(3): e2006532.
- Xiong, X., Xu, Z., Huang, H., Wang, Y., Zhao, J., Guo, X. and Zhou, S. (2020). A NIR light triggered disintegratable nanoplatform for enhanced penetration and chemotherapy in deep tumor tissues. Biomaterials 245: 119840.
- Yan, F., Duan, W., Li, Y., Wu, H., Zhou, Y., Pan, M., Liu, H., Liu, X. and Zheng, H. (2016). NIR-Laser-Controlled Drug Release from DOX/IR-780-Loaded Temperature-Sensitive-Liposomes for Chemo-Photothermal Synergistic Tumor Therapy. Theranostics 6(13): 2337-2351.
- Li, H. J., Du, J. Z., Liu, J., Du, X. J., Shen, S., Zhu, Y. H., Wang, X., Ye, X., Nie, S. and Wang, J. (2016). Smart Superstructures with Ultrahigh pH-Sensitivity for Targeting Acidic Tumor Microenvironment: Instantaneous Size Switching and Improved Tumor Penetration. ACS Nano 10(7): 6753-6761.
- Wang, G., Zhou, Z., Zhao, Z., Li, Q., Wu, Y., Yan, S., Shen, Y. and Huang, P. (2020). Enzyme-Triggered Transcytosis of Dendrimer-Drug Conjugate for Deep Penetration into Pancreatic Tumors. ACS Nano 14(4): 4890-4904.
- Zhou, Q., Shao, S., Wang, J., Xu, C., Xiang, J., Piao, Y., Zhou, Z., Yu, Q., Tang, J., Liu, X., et al. (2019). Enzyme-activatable polymer-drug conjugate augments tumour penetration and treatment efficacy. Nat Nanotechnol 14(8): 799-809.
- Zhang, Y., Cui, Y., Li, M., Cui, K., Li, R., Xie, W., Liu, L. and Xiao, Z. (2022). DNA-assembled visible nanodandelions with explosive hydrogen-bond breakage achieving uniform intra-tumor distribution (UITD)-guided photothermal therapy. Biomaterials 282: 121381.
Article Information
Copyright
© 2023 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wang, H., Cui, Y., Zhang, Y. and Xiao, Z. (2023). Preparation and Characterization of DNA-assembled GRS-DNA-CuS Nanodandelions. Bio-protocol 13(2): e4595. DOI: 10.21769/BioProtoc.4595.
Category
Molecular Biology > Nanoparticle > Plan-derived nanoparticles
Biological Engineering > Biomedical engineering
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









