- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
PERK Pathway Inhibitors Cure Group A Streptococcal Necrotizing Fasciitis in a Murine Model
(*contributed equally to this work) Published: Vol 12, Iss 24, Dec 20, 2022 DOI: 10.21769/BioProtoc.4589 Views: 2122
Reviewed by: Luis Alberto Sánchez VargasKarolina SubrtovaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Murine Acute Pneumonia Model of Pseudomonas aeruginosa Lung Infection
Xiaolei Pan and Weihui Wu
Nov 5, 2020 4640 Views
A Retro-orbital Sinus Injection Mouse Model to Study Early Events and Reorganization of the Astrocytic Network during Pneumococcal Meningitis
Chakir Bello [...] Guy Tran Van Nhieu
Dec 5, 2021 3377 Views
Rapid in vitro and in vivo Evaluation of Antimicrobial Formulations Using Bioluminescent Pathogenic Bacteria
Artur Schmidtchen and Manoj Puthia
Jan 20, 2022 3903 Views
Abstract
Group A streptococcus (GAS) is a Gram-positive human pathogen that causes invasive infections with mild to life-threatening severity, like toxic shock syndrome, rheumatic heart disease, and necrotizing fasciitis (NF). NF is characterized by a clinical presentation of widespread tissue destruction due to the rapid spread of GAS infection into fascial planes. Despite quick medical interventions, mortality from NF is high. The early onset of the disease is difficult to diagnose because of non-specific clinical symptoms. Moreover, the unavailability of an effective vaccine against GAS warrants a genuine need for alternative treatments against GAS NF. One endoplasmic reticulum stress signaling pathway (PERK pathway) gets triggered in the host upon GAS infection. Bacteria utilize asparagine release as an output of this pathway for its pathogenesis. We reported that the combination of sub-cutaneous (SC) and intraperitoneal (IP) administration of PERK pathway inhibitors (GSK2656157 and ISRIB) cures local as well as systemic GAS infection in a NF murine model, by reducing asparagine release at the infection site. This protocol's methodology is detailed below.
Background
Group A Streptococcus (GAS), or Streptococcus pyogenes , is a human-specific Gram-positive bacterial pathogen. It remains among the top ten pathogens causing several diseases with mild to life-threatening clinical symptoms (Bisno and Stevens, 1996;Efstratiou and Lamagni, 2016). The clinical presentation of these diseases includes pharyngitis, impetigo, erysipelas, tonsilitis, scarlet fever, necrotizing fasciitis, rheumatic fever, glomerulonephritis, septicemia, and others. More than 220 different GAS serotypes are circulating in the human population worldwide. M1T1 is the dominant serotype spread globally and causing diseases in the population of economically well-equipped countries (Aziz and Kotb, 2008; Nelson et al., 2016; Lynskey et al., 2019). GAS classification is based on variation in the N-terminal amino acid sequence of the surface M-protein. Antibiotic regimens are the only definitive strategy to cure GAS infections, because of the unavailability of any licensed universal vaccine on the market. Recent reports on the development of antibiotic resistance in GAS are worrisome and have motivated us to develop a new alternative therapeutic approach to cure its infections.
Invasive GAS infections, like sepsis, bacteremic pneumonia, and necrotizing fasciitis (NF), spread in sterile sites (Metzgar and Zampolli, 2011). NF begins as a small lesion with the appearance of mild erythema, which rapidly progresses with inflammation in the subcutaneous tissue, and destructs skin and soft tissue over large body areas (Walker et al., 2014). Antibiotic administration, surgical debridement of infected tissues, and supportive care are the main line of treatment for invasive GAS diseases (Stevens and Bryant, 2017). Unfortunately, the mortality rate from NF ranges from 23 to 35% in resource-rich settings, despite quick medical interventions (Davies et al., 1996; Carapetis et al., 2005;Allen and Moore, 2010;Olsen and Musser, 2010; Cole et al., 2011;Ralph and Carapetis, 2013). Therefore, alternative therapeutic approaches are urgently needed to combat invasive soft-tissue GAS infections.
Our recent studies showed that GAS causes endoplasmic reticulum stress (ER stress) and triggers the unfolded protein response (UPR) in the host cells upon infection. This results in enhanced asparagine synthesis and release, which is utilized by GAS to increase virulence and rate of proliferation. Furthermore, the PERK-eIF2α-ATF4 branch of UPR was exclusively triggered during GAS infection (Anand et al., 2021). When we treated GAS-infected mice with PERK pathway inhibitors (GSK2656157 and ISRIB), the bacteria were cleared faster than in untreated infected mice. We discuss the method of treatment using PERK pathway inhibitors in a murine model of human soft tissue infection.
Materials and reagents
Preparation of bacteria for mice infection
15 mL conical tubes (Greiner Bio-One CELLSTAR, catalog number: 188621)
50 mL conical tubes (MiniPlast, catalog number: 835-050-21-111)
Microcentrifuge tubes (Corning® Axygen, catalog number: MCT-175-C)
Spectrophotometric cuvettes (Polystyrene cuvette, 10 × 4 × 45 mm, Sarstedt AG & Co. KG, Germany, catalog number: 67.742)
GAS strains (serotype M14 strain JS14, which was isolated from a patient with pneumonia, and serotype M1T1 strain 5448, a clinical isolate from a patient with necrotizing fasciitis and toxic shock syndrome)
Sterile Dulbecco's Phosphate Buffered Saline (without calcium chloride and magnesium chloride, pH 7.1–7.5, Biological Industries, catalog number: 02-023-1A)
THY media (see Recipes)
Todd-Hewitt broth (Becton, Dickenson, and Company, catalog number: 249240)
Yeast extract (Becton, Dickenson, and Company, catalog number: 212750)
Blood Agar plates (see Recipes)
Defibrinated sheep blood (DSB) (hylabs®, catalog number: PD049)
Mice infection
1 mL syringes without needles (BD PlastipakTM 1 mL Luer, catalog number: 303172)
Needle (30G × 1/2") for syringe (BD MicrolanceTM 3, catalog number: 2025-01)
Bacterial culture prepared for injection (suspended in PBS)
Female BALB/c OlaHsd mice, aged 3–4 weeks, weighing 10–12 g (ENVIGO RMS, Israel Ltd.)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 472301)
Polyethylene glycol (PEG-400) (Sigma-Aldrich, catalog number: 202398)
0.9% sodium chloride saline (Teva Medical, catalog number: AWN1324)
Ketamine-xylazine mix (mixed in 1:6 ratio, respectively, Ketamine-Vetoquinol, Xylazine-20 mg/mL, Eurovet Animal Health B.V.)
Isoflurane (Piramal Critical Care Inc, USA, catalog number: 66794-013-25)
GSK2656157 (Sigma-Aldrich, catalog number: 504651) (see Recipes)
Integrated stress response inhibitor (ISRIB) (Sigma-Aldrich, catalog number: SML0843) (see Recipes)
CFU quantification of GAS in skin biopsy samples
Sterile 2 mL tubes (Abdos Labtech Private Limited, catalog number: P10203)
Sterile Dulbecco's Phosphate Buffered Saline (without calcium chloride and magnesium chloride, pH 7.1–7.5, Biological Industries, catalog number: 02-023-1A)
70% Ethanol (Romaical, 19-19-009102-80)
8.0-mm Punch biopsy (Acu-Punch, Acuderm Inc., catalog number: P825)
Dissection kit—surgical scissors and fine forceps
Blood Agar plates (see Recipes)
Defibrinated sheep blood (DSB) (hylabs®, catalog number: PD049)
Equipment
Spectrophotometer (Amersham Biosciences, Ultraspec 2100 pro, 80-2112-21)
Centrifuge with swing rotor (Sigma, model: 3-16PK)
Vortex mixer (Heidolph reax top, Z655988)
-80 °C freezer (Thermo Scientific, 8926)
37 °C incubator (BINDER GmbH, BD 56)
Digital vernier caliper (Bar Naor Ltd., BN30087-00)
Electronic homogenizer (POLYTRON® 2100 Homogenizers, Kinematica, 05400261)
Electric shaver (Phillips, MG3730/15)
Desiccator jar (SP BEL-ART polycarbonate/polypropylene desiccator, F42032-0000)
Software
GraphPad Prism 5.03 (for statistical analysis and graphical presentation)
Procedure
Preparation of bacteria for mice infection
From a frozen glycerol stock (bacteria grown overnight were frozen in 15% v/v final strength sterile glycerol), inoculate the GAS strain into 10 mL of THY medium in 15 mL sealed conical tubes. Culture the bacteria statically at 37 °C for 16 h.
Transfer 3 mL of overnight culture into 47 mL of pre-warmed fresh THY medium in 50 mL sealed conical tubes. Grow the bacteria at 37 °C without agitation to the mid-log phase, until the optical density at 600 nm (OD600 ) reaches 0.3–0.4.
Centrifuge culture tubes at 4,000 × g and 14 °C for 10 min, and discard the supernatant. Wash the bacterial pellet after suspending it in 20 mL of sterile PBS. Centrifuge at 4,000 × g and 14 °C for 10 min. Repeat the washing step twice. Discard the supernatant and resuspend the bacterial pellet in 1 mL of sterile PBS.
Measure the OD600 of the bacterial suspension and adjust it with PBS to 0.8 (corresponding to 1 × 109 CFU/mL). Dilute the bacterial culture 1:20 in PBS to get 5 × 107 CFU/mL (sub-lethal dose). Place the tubes on ice until use.
CFU counts are determined to calculate the number of bacteria injected into the mice. First, serially dilute the bacterial inoculum to seven dilutions, by adding 100 µL of inoculum to 900 µL of PBS. Next, plate 100 µL of the 10-5 , 10-6 , and 10-7 dilutions onto blood agar plates. Finally, incubate the plates at 37 °C overnight.
To determine the CFU/mL, count the number of colonies on the blood agar plates in the appropriate dilution and multiply by the dilution factor.
In vivo soft-tissue model
Three to four weeks old female BALB/c OlaHsd mice (weighing 10–12 g) were left to acclimatize for three days in disposable cages supplemented with enrichment, sterile food, and water, in specific pathogen-free (SPF) rooms with controlled environmental conditions.
Weigh mice and distribute them evenly in cages. Mark mice on ears and shave their dorsal flanks one day before bacterial infection.
Different groups of mice receive 100 µL of subcutaneous (SC) or intraperitoneal (IP) injections of GSK2656157 (100 µg/mouse/injection) or ISRIB (35 µg/mouse/injection) or control (90 µL of saline + 10 µL of DMSO and PEG 400 at 1:1 ratio).
Inject GSK2656157/ISRIB subcutaneously 4 h before bacterial infection. Subsequently, SC and IP injections of the inhibitors are alternately given at an interval of 12 h post-infection (PI) until the sixth day. In the post-treatment mice experiment, inject GSK2656157/ISRIB subcutaneously for 12 h PI, followed by IP and SC injections of GSK2656157/ISRIB, which are given alternately at an interval of 12 h until the sixth day (Figure 1).
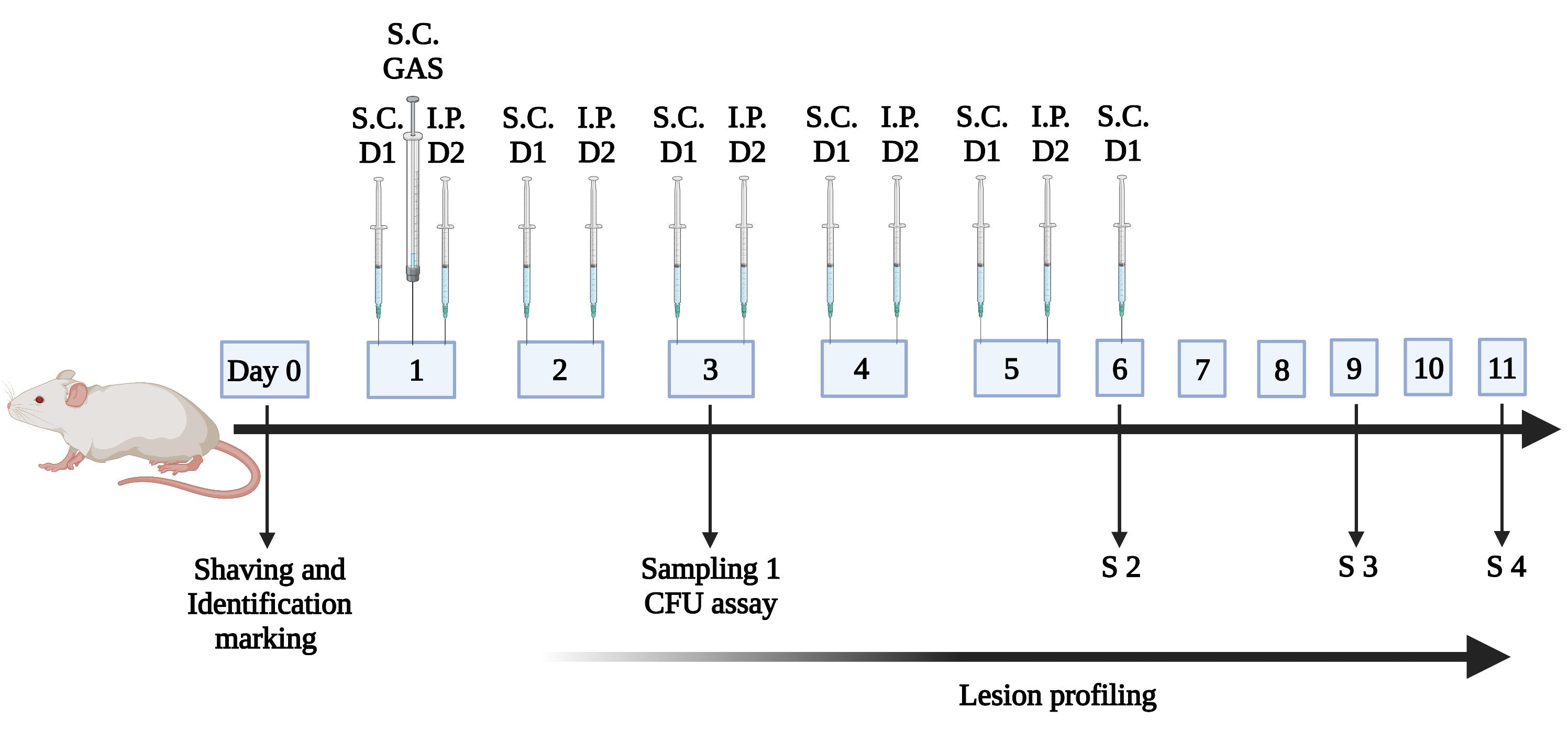
Figure 1. Timeline of the injection of inhibitors and sampling procedure.BALB/c mice were placed in specific pathogen-free (SPF) conditions with controlled environmental conditions. They were weighed, shaved on dorsal flanks, and marked for identification. Mice were infected by subcutaneous (SC) administration of 5 × 106 CFU of GAS strain, and were either untreated, treated 4 h before infection, or treated 12 h post-infection (PI) with GSK2656157/ISRIB. The inhibitors were injected every 12 h alternately with dose 1 (D1) and dose 2 (D2) through SC or intraperitoneal (IP) routes for 6 days. Sampling (S1, S2, S3, and S4) for quantification of bacteria through skin biopsy was performed at different time intervals.
Inject mice with a sub-lethal dose (5 × 106 CFU) of GAS to test the efficacy of GSK2656157/ISRIB. A volume of 100 µL of the bacterial solution is injected SC into the rear flank of mice. The bacterial load is confirmed by serial dilution and CFU estimation on blood agar plates.
Euthanize mice by inhalation of isoflurane followed by cervical dislocation, and collect skin tissue samples using an 8.0-mm punch biopsy tool at different time points. Transfer the tissue samples to 2 mL Eppendorf tubes containing 0.5 mL of sterile PBS. Homogenize skin tissue samples using a homogenizer at a vibration speed of 20,000 ± 100 rpm for 30 s, dilute this in PBS, and plate the samples on blood agar plates. CFUs are quantified after incubation of blood agar plates at 37 °C overnight. The amount of CFU is normalized to the weight of the soft tissue sample.
Measure the dermonecrotic lesion area of the mice in different groups daily, using a digital vernier caliper. The lesion area is calculated with the formula A = π/2)(length)(width) (Pinho-Ribeiro et al., 2018). The maximum permissible dermonecrotic lesion area is 2 cm2 .
All the quantitative data (CFU counts and dermonecrotic lesion area) can be tested for statistical significance using GraphPad Prism 5.03 software.
Notes
The mice should be checked twice daily, including weighing.
To minimize the suffering of the mice, a scoring method needs to be employed based on the status of their hair (straight or ruffled coat), eyes (open or closed), the activity of the mice, weight loss (loss of 15% body weight between two consecutive measurements), and severity of the lesion.
Euthanize mice by injecting an overdose of ketamine/xylazine mix (total of 60 µL in a ratio of 1:6, respectively), followed by cervical dislocation, when scoring points to the need of an early humane endpoint.
Recipes
THY media
Todd-Hewitt broth (3%) supplemented with 0.2% yeast extract in distilled water
Blood Agar plates
Tryptic soy agar (TSA) supplemented with 5% (v/v) of defibrinated sheep blood (DSB)
GSK2656157
Dissolve GSK2656157 in DMSO, and add an equal volume of PEG 400.
The aliquots can be stored at -80 °C until use (to be used within 3 months). On the day of injection, the mixture is diluted 10 times in saline (0.9% sodium chloride) to achieve a final concentration of 1 mg/mL.
ISRIB
Dissolve ISRIB in DMSO, and add an equal volume of PEG 400.
The aliquots can be stored at -80 °C until use (to be used within 3 months). On the day of injection, the mixture is diluted 10 times in saline (0.9% sodium chloride) to achieve a final concentration of 0.35 mg/mL.
Acknowledgments
This work was supported by grants from the Israeli Science Foundation administered by the Israel Academy of Sciences and Humanities (ISF), the FIRST grant from ISF, the ISF-UGC Joint Scientific Research Program, and the National Research Foundation, Prime Minister’s Office, Singapore under its Campus of Research Excellence and Technological Enterprise (CREATE) program. In addition, AA and AS are thankful to the PBC Fellowship Program by the Israeli council for partial funding. This protocol was used in Anand et al. (2021) (DOI: 10.1126/scitranslmed.abd7465).
Competing interests
The authors declare no conflict of interest or competing interests.
Ethics
All the animal procedures for humane handling, care, and treatment of research animals are performed according to the ethical guidelines approved by the ethics committee of the Hebrew University of Jerusalem (Protocol number MD-18-15522-5).
References
- Bisno, A. L. and Stevens, D. L. (1996). Streptococcal infections of skin and soft tissues. N Engl J Med 334(4): 240-245.
- Efstratiou, A. and Lamagni, T. (2016). Epidemiology of Streptococcus pyogenes. Ferretti, J. J., Stevens, D. L. and Fischetti, V. A. (Eds.) Streptococcus pyogenes: Basic Biology to Clinical Manifestations.
- Aziz, R. K. and Kotb, M. (2008). Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg Infect Dis 14(10): 1511-1517.
- Nelson, G. E., Pondo, T., Toews, K. A., Farley, M. M., Lindegren, M. L., Lynfield, R., Aragon, D., Zansky, S. M., Watt, J. P., Cieslak, P. R., Angeles, K., Harrison, L. H., Petit, S., Beall, B. and Van Beneden, C. A. (2016). Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis 63(4): 478-486.
- Lynskey, N. N., Jauneikaite, E., Li, H. K., Zhi, X., Turner, C. E., Mosavie, M., Pearson, M., Asai, M., Lobkowicz, L., Chow, J. Y., et al. (2019). Emergence of dominant toxigenic M1T1 Streptococcus pyogenes clone during increased scarlet fever activity in England: a population-based molecular epidemiological study. Lancet Infect Dis 19(11): 1209-1218.
- Metzgar, D. and Zampolli, A. (2011). The M protein of group A Streptococcus is a key virulence factor and a clinically relevant strain identification marker. Virulence 2(5): 402-412.
- Walker, M. J., Barnett, T. C., McArthur, J. D., Cole, J. N., Gillen, C. M., Henningham, A., Sriprakash, K. S., Sanderson-Smith, M. L. and Nizet, V. (2014). Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin Microbiol Rev 27(2): 264-301.
- Stevens, D. L. and Bryant, A. E. (2017). Necrotizing Soft-Tissue Infections. N Engl J Med 377(23): 2253-2265.
- Davies, H. D., McGeer, A., Schwartz, B., Green, K., Cann, D., Simor, A. E. and Low, D. E. (1996). Invasive group A streptococcal infections in Ontario, Canada. Ontario Group A Streptococcal Study Group. N Engl J Med 335(8): 547-554.
- Carapetis, J. R., Steer, A. C., Mulholland, E. K. and Weber, M. (2005). The global burden of group A streptococcal diseases. Lancet Infect Dis 5(11): 685-694.
- Allen, U. and Moore, D. (2010). Invasive group A streptococcal disease: Management and chemoprophylaxis. Can J Infect Dis Med Microbiol 21(3): 115-118.
- Olsen, R. J. and Musser, J. M. (2010). Molecular pathogenesis of necrotizing fasciitis. Annu Rev Pathol 5: 1-31.
- Cole, J. N., Barnett, T. C., Nizet, V. and Walker, M. J. (2011). Molecular insight into invasive group A streptococcal disease. Nat Rev Microbiol 9(10): 724-736.
- Ralph, A. P. and Carapetis, J. R. (2013). Group a streptococcal diseases and their global burden. Curr Top Microbiol Immunol 368: 1-27.
- Anand, A., Sharma, A., Ravins, M., Biswas, D., Ambalavanan, P., Lim, K. X. Z., Tan, R. Y. M., Johri, A. K., Tirosh, B. and Hanski, E. (2021). Unfolded protein response inhibitors cure group A streptococcal necrotizing fasciitis by modulating host asparagine. Sci Transl Med 13(605).
- Pinho-Ribeiro, F. A., Baddal, B., Haarsma, R., O'Seaghdha, M., Yang, N. J., Blake, K. J., Portley, M., Verri, W. A., Dale, J. B., Wessels, M. R., et al. (2018). Blocking Neuronal Signaling to Immune Cells Treats Streptococcal Invasive Infection. Cell 173(5): 1083-1097 e1022.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Anand, A., Sharma, A., Ravins, M., Johri, A. K., Tirosh, B. and Hanski, E. (2022). PERK Pathway Inhibitors Cure Group A Streptococcal Necrotizing Fasciitis in a Murine Model. Bio-protocol 12(24): e4589. DOI: 10.21769/BioProtoc.4589.
- Anand, A., Sharma, A., Ravins, M., Biswas, D., Ambalavanan, P., Lim, K. X. Z., Tan, R. Y. M., Johri, A. K., Tirosh, B. and Hanski, E. (2021). Unfolded protein response inhibitors cure group A streptococcal necrotizing fasciitis by modulating host asparagine. Sci Transl Med 13(605).
Category
Microbiology > in vivo model > Bacterium
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









