- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation of Caenorhabditis elegans for Scoring of Muscle-derived Exophers
Published: Vol 13, Iss 1, Jan 5, 2023 DOI: 10.21769/BioProtoc.4586 Views: 1861
Reviewed by: Andrea PuharRama Reddy GoluguriAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Fixation and Immunostaining of Endogenous Proteins or Post-translational Modifications in Caenorhabditis elegans
Robert O'Hagan and Irini Topalidou
Oct 5, 2021 3823 Views
Labelling of Active Transcription Sites with Argonaute NRDE-3—Image Active Transcription Sites in vivo in Caenorhabditis elegans
Antoine Barrière and Vincent Bertrand
Jun 5, 2022 2683 Views
Monitoring the Recruitment and Fusion of Autophagosomes to Phagosomes During the Clearance of Apoptotic Cells in the Nematode Caenorhabditis elegans
Omar Peña-Ramos and Zheng Zhou
Nov 20, 2022 2131 Views
Abstract
Utilizingresources available from the mother's body to guarantee healthy offspring growth is the fundamental reproductive strategy. Recently, we showed that a class of the largest extracellular vesicles known as exophers, which are responsible for the removal of neurotoxic components from neurons (Melentijevic et al., 2017) and damaged mitochondria from cardiomyocytes (Nicolás-Ávila et al., 2020), are released by the Caenorhabditis elegans hermaphrodite body wall muscles (BWM), to support embryonic growth (Turek et al., 2021). Employing worms expressing fluorescent reporters in BWM cells, we found that exopher formation (exophergenesis) is sex-specific and fertility-dependent. Moreover, exophergenesis is regulated by the developing embryo in utero, and exophers serve as transporters for muscle-generated yolk proteins, which can be used to nourish the next generation. Given the specific regulation of muscular exophergenesis, and the fact that muscle-generated exophers are much larger than neuronal ones and have different targeting, their identification and quantification required a modified approach from that designed for neuronal-derived exophers (Arnold et al., 2020). Here, we present a methodology for assessing and quantifying muscle-derived exophers that can be easily extended to determine their function and regulation in various biological contexts.
Graphical abstract

Background
Maintaining protein homeostasis (proteostasis) requires the degradation of damaged or unwanted proteins and plays a key role in the function of cells and organisms. The main proteolytic component of the cellular proteostasis network is the ubiquitin-proteasome system (UPS), by which protein substrates are labelled by attachment of a small ubiquitin protein and then targeted for degradation by the proteasome. In addition, the autophagy-lysosome pathway promotes proteostasis through the turnover of aggregated proteins and obsolete organelles (Mizushima and Komatsu, 2011; Gatica et al., 2018). Recently, a novel proteostasis mechanism was described in Caenorhabditis elegans , where protein aggregates, mitochondria, and lysosomes are removed from worm neurons to the hypodermis via large vesicles (of approximately 4 μm in diameter) called exophers (Melentijevic et al., 2017). Exophers are generated independently of the endosomal sorting complexes required for transport (ESCRT) machinery, and their surface lacks phosphatidylserine, distinguishing them from exosomes or apoptotic bodies. Conditions that interfere with proteostasis, and when protein turnover or autophagy is inhibited, increase exopher production. Exophers are sorted in the ced-1, ced-6, and ced-7 engulfment pathways (Melentijevic et al., 2017). Exophergenesis is evolutionarily conserved, as the malfunctioning mitochondria are excreted via exophers by mouse cardiomyocytes (Nicolás-Ávila et al., 2020), and exophers were identified in the human and mice neurons (Siddique et al., 2021). However, the biological function of exophers is not limited to eliminating cellular waste. We have demonstrated that the body wall muscle (BWM) of C. elegans can expel cellular content via robust exophers, which is later used to nourish the next generation. We identified these using the worms’ strains with fluorescently labeled mitochondria (GFP anchored in the mitochondrial membrane through a sequence of 50 aa of TOMM-20) and proteasome subunits, RPN-5 (19S subunit), PAS-7, or PAS-4 (20S subunits), as we initially studied exophers with regard to protein turnover. Muscle exophers, like their neuronal counterparts, are jettisoned from the cell body. Some remain connected to the extruding cell by a flexible tube that permits the transfer of cellular material to the attached vesicle (Figure 1A). Muscle exophers can also contain mitochondria, which in electron microscopy (EM) images showed increased surface area and disrupted cristae organization, as in cardiac exophers. Moreover, muscular exophergenesis is also not active during the larval stages, and its maximum level is reached around the second and third day of hermaphrodite adulthood (Turek et al., 2021). In addition, its activity is also reduced by the depletion of NADPH-cytochrome P450 reductase EMB-8 and actin-binding protein POD-1 (Melentijevic et al., 2017). However, in contrast to neurons or cardiomyocytes, we observed a low number of mitochondria-containing vesicles and a lack of significant changes in exophergenesis in response to proteotoxic stress. Our further analyses showed that BWM exophers represent a transgenerational metabolic/resource management system induced by the appearance of developing embryos in utero. In response, the newly formed exophers transport muscle-generated yolk proteins that can support the development of the offspring. Our data also suggests that BWM exophers are controlled by signals from developing embryos (Turek et al., 2021). Given the above, and that BWM exophers are generally more prominent in size than neuronal ones (up to 15 µm in diameter) and extruded into the body cavity, their identification, quantification, and analysis requires a modified methodology from that described for exophers of neuronal origin (Arnold et al., 2020). Here we provide details on the strains of C. elegans , time and conditions of growth, and a step-by-step procedure for imaging and quantifying BWM exophers. We believe this protocol will allow other laboratories to easily adapt their resources to decipher the autonomous and non-autonomous regulation of the BWM exophergenesis.
Materials and Reagents
Pipettes and pipette tips (Eppendorf)
1.5 mL and 50 mL polypropylene conical tube (VWR)
Fluorescent reporters produced from body wall muscle promoter myo-3 that label cytoplasmic proteins, and/or organelles (mitochondria or lysosomes) that will be excreted in exophers can be used to visualise and measure the exophergenesis. We commonly use ACH93 or ACH81 worm strains, where wrmScarlet is fused to proteasome subunit (RPN-5), and GFP is tagged to the TOMM-20 mitochondrial protein of the outer membrane, allowing for exopher visualization (wrmScarlet fluorescent signal) and inspection of mitochondria presence (GFP fluorescent signal) (Figure 1B–1C). We have prepared the following protocol for the C. elegans strains we use most often (Table 1), but this procedure will also be effective for other lines, as, those based on cytoplasmic GFP produced in BWM (Pmyo-3::GFP), which is also present in released exophers.
Table 1. Strains we use to study muscle-derived exophers
C. elegans strain Description Source ACH93 wacIs1[myo-3p::rpn-5 CAI = 0.97::GGGGS Linker-wrmScarlet::unc-54 3′UTR, unc-119(+)], wacIs14[myo-3 promoter::tomm-20_1–50aa::attB5::mGFP::unc-54-3′UTR, unc-119(+)] Turek et al. (2021) ACH81 wacIs1[myo-3 promoter::rpn-5 CAI=0.97::Optimal Linker-wrmScarlet::unc-54 3’UTR, unc-119(+)] Turek et al. (2021) 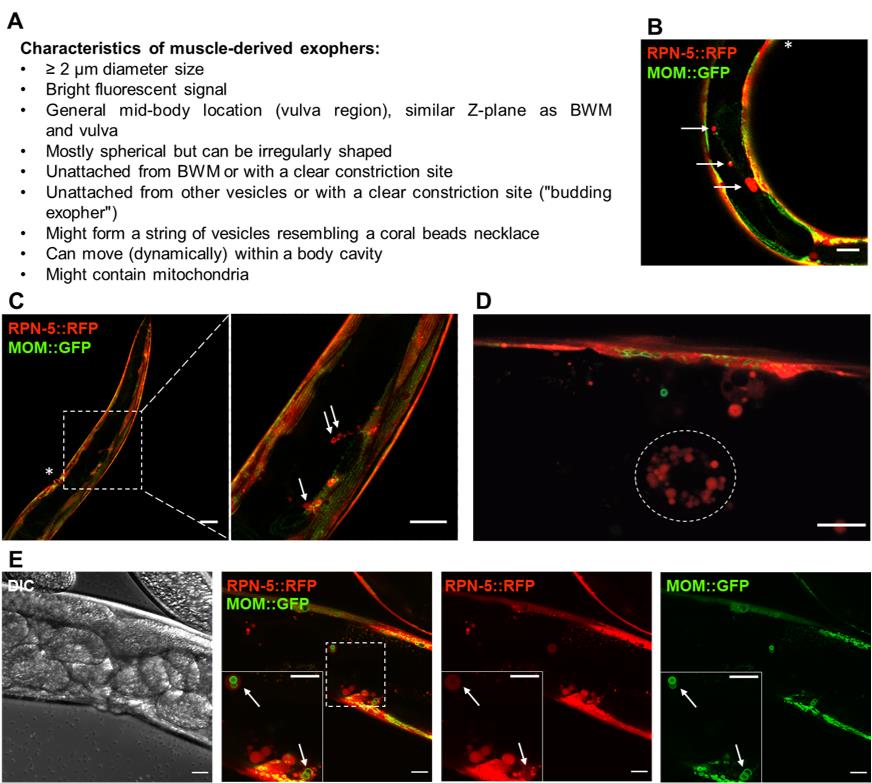
Figure 1. Fluorescently labelled (wrmScarlet) exophers extruded from the body wall muscles on day 2 of adulthood. A. Characteristics of muscle-derived exophers in C. elegans . B–C. Cross-sections of ACH93 worms (with a focus on the midbody) visualized in the RFP and GFP channels of the confocal microscope (Zeiss LSM800). The square region on panel C marked with a dashed line is magnified 3.2× on its right side. The arrows point to exophers, and the asterisks indicate the position of the vulva. Structures of an irregular, non-round shape or inside the BWM are not classified as exophers. Scale bars are 20 μm in B and 50 and 20 μm in C. D. The wrmScarlet signal may also originate from scattered and smaller vesicles (< 2 μm) present in the body cavity (circled with a dotted line). In our readings, we do not count these structures as exophers, as these are coelomocytes that degrade fluorescent proteins taken up from a pseudocoelom. Scale bar is 10 µm. E. Zoom into the vulva region in separate channels: DIC, a merge of RFP and GFP, separate RFP and GFP. The square marked with a dashed line marks the boundaries of the enlarged region in the bottom left corner. The arrows indicate exophers with mitochondria. MOM—mitochondrial outer membrane. Scale bar is 20 µm.LB broth (Sigma, catalog number: L3022-1KG)
Na2HPO4 (Sigma-Aldrich, catalog number: S7907)
KH2PO4 (Roth, catalog number: 3904.1)
NaCl (Chempur, catalog number: 117941206)
MgSO4 (Sigma-Aldrich, catalog number: M5921)
Peptone (BioShop, catalog number: PEP403.1)
Agar (BioShop, catalog number: AGR001.1)
KPO4 (Roth, catalog number: P749.1)
Streptomycin (Sigma-Aldrich, catalog number: S6501)
Nystatin (Sigma-Aldrich, catalog number: N1638)
Tetramisole hydrochloride (Sigma-Aldrich, catalog number: L9756)
Escherichia coli OP50 strain or any other bacteria that can serve as a food source (obtained from the Caenorhabditis Genetics Center, CGC) (see Recipes)
M9 buffer (see Recipes)
Nematode growth medium (NGM) (see Recipes)
Equipment
Fluorescence stereo or confocal microscope that allows for at least 200× magnification (ZEISS Axio Zoom.V16 Motorized Fluorescence Stereo Zoom Microscope)
Incubator for C. elegans (Q-Cell, PolLab)
Platinum home-made wire worm pick
Alcohol burner (DWK Life Sciences Wheaton)
Petri dishes for C. elegans cultivation (VWR)
Software
GraphPad Prism 9.3.1 (GraphPad Software)
Excel 2019 (Microsoft Office)
ZEN (Zeiss)
Procedure
Maintenance of C. elegans
Since exophergenesis is a process that is influenced by food availability, make sure you provide constant, optimal conditions of worms’ culture:
Keep worms at 20 °C for at least 2–3 generations before running an experiment (a necessary step, especially after thawing animals from -80 °C stocks).
The authors avoid bleaching of gravid C. elegans followed by a short period of starvation of L1 larvae, to avoid the potential effects of bleach and food deficiency on exophergenesis in later stages of development. Instead, transfer gravid worms (or at any other desired developmental stage) to fresh NGM plates with bacterial food using a sterile platinum worm pick, or wash them off from old plates with M9 buffer and seed them using a pipette under sterile conditions. If you decide to transfer with M9, wash animals three times in the Eppendorf tube, to avoid contamination of a new plate with bacteria from the previous plate. Centrifugation at 2,000–3,000 rpm is permitted.
Make sure worms have access to food ad libitum. Starvation severely affects exophergenesis. If you starve animals, maintain them for 2–3 generations with constant access to a food source before starting a new experiment.
Minimize the risk of contamination by following good laboratory practices, wearing gloves, disinfecting the work surface, working beside the burner, sterilising the worm pick, etc. If you notice contamination (e.g., spots of other bacteria, or fungi on plates), discard the plate, and thaw a fresh stock of reporter strain. Otherwise, try to transfer eggs laid as far from the contamination as possible to an empty NGM plate, wait until they hatch, and transfer single worms to a fresh plate, preferably at the edge, where there is no bacterial food. Again, such worms should not be used for experiments before ensuring contamination is eliminated.
Setup of worms for scoring exophers
Obtain a synchronized population of either:
Eggs—transfer approximately 30–50 gravid worms with a worm pick to a fresh plate, allow them to lay eggs for 2–3 h (in the 20 °C incubator), and then remove adult worms. Alternatively, from a plate that contains mixed-stage worms, transfer eggs that contain 3-fold embryos (pretzel stage embryo) to a fresh plate.
Freshly hatched L1 larvae—remove all larvae and adult worms from plate(s) with an unsynchronized population (by washing them off with M9), leaving only eggs on a plate. Place plate(s) in the incubator for 30 min–2 h (depending on how many eggs were initially on the plate, and how many are needed for your experiment; the shorter the period of incubation, the better synchronized the population will be), and allow them to hatch in the meantime.
Once you obtain enough synchronized eggs (method a) or freshly hatched L1s (method b), transfer them to fresh plates. Try to maintain a similar number of worms per plate. For experiments with sufficient statistical power, seeding 50 worms per 60 mm Petri dish is optimal. If you seed more, you risk insufficient access to the food throughout the experiment. Avoid plates with fluoro-deoxyuridine (FUdR), which inhibits exopher production. Conversely, less than 20 worms per plate would require more than three biological repeats to reliably visualize the differences between the groups. Maintain worms at 20 °C unless your hypothesis requires otherwise.
Synchronous growth of larvae at later stages
Monitor if the worms develop synchronously again at the L4 larval stage. Take the development of the vulva and its morphological differences in shape defined in subsequent sub-stages as a benchmark (Mok et al., 2015). To provide the best possible result reproducibility, sort worms at L4.5–L4.9 (approximately 43–46 h from hatching). This timing might require adjustment due to seasonal weather changes, if you do not use air conditioning set to a constant temperature throughout the year. Remove worms in other developmental stages. To ensure you do not miss any, you can transfer single worms of interest to fresh plates using a worm pick.
Scoring exophers at adulthood day 2
The exophergenesis peak coincides with the highest egg-laying activity, as shown in Turek et al. (2021). The egg-laying behaviour of the hermaphrodite occurs ~65–128 h from hatching (Altun and Hall, 2012 in WormAtlas), with the maximal egg-laying at approximately 96 h. Therefore, for the highest reproducibility of the results, we score exophers on adulthood day 2, approximately 98 h (± 2 h) from hatching into L1 larvae.
When worms reach the desired developmental stage, visualize them under the fluorescence microscope. At first, monitor their general fitness using brightfield—if the majority of control or single worms from the experimental group express abnormalities, do not use them for the research. Discard plates with contamination if not done earlier. A fluorescence stereomicroscope was used to score exophers in freely moving animals directly on NGM plates. We used a ZEISS Axio Zoom.V16 Motorized Fluorescence Stereo Zoom Microscope equipped with HXP 200 C fluorescence lamp (Kübler). For ACH81 and ACH93 strains, we used the Texas Red filter set to visualize wrmScarlet (wrmScarlet is fused to proteasome subunits of the BWM), and the GFP filter set (GFP is tagged to the mitochondrial outer membrane of the BWM). The GFP channel is not required if you are interested in the number of exophers, but not on the distribution of mitochondria.
To acquire images with a confocal microscope, transfer worms onto freshly prepared 3% agarose (in H2O) pads on a microscope slide. Next, immobilize them with 25 μM tetramisole/levamisole and cover them with a round or square glass coverslip. We used an inverted Zeiss LSM 800 laser-scanning confocal microscope with a 40× oil objective, equipped with 488-nm and 561-nm lasers, to excite the GFP and RFP fluorescent proteins, respectively. To investigate the presence and distribution of exophers, collect Z-stacks and process them with ZEN (Zeiss) or ImageJ (Fiji) software.
Score the exophers on the ≥ 200 magnification under a fluorescence stereomicroscope. You will see the biggest vesicles even at lower magnification (16×, objective 1.0), but your quantification will not be precise (Figure 1C , left panel). Most exophers will be located near the vulva region, but can also occur anywhere in the body cavity.
Exophers are round vesicles that vary in size (Figure 1A). However, sometimes they remain attached to a releasing cell (Figure 1B–1C). Reject small vesicles with weak fluorescence that do not reach ~1/10 of the width of the BWM cell in quantifying exophergenesis, as well as any other irregular shaped fluorescent structures in the body cavity (Figure 1D).
Data are collected as the number of exophers visible in a single worm from one experimental/control group. (e.g., if you have 40 animals on the plate, you should obtain 40 counts). Microsoft Excel is handy for the storage of the data generated this way. Maintain separate sheets for each biological repetition, to quickly detect any disturbances.
Once you collect data from all repeats, we recommend you copy them to GraphPad Prism for further processing (statistical analysis, graphical visualization, Figure 2).
Data analysis
Each experiment consists of three independent biological replicates. We assume non-Gaussian distribution of residuals, and therefore apply nonparametric statistical tests: Mann–Whitney (when we compare only two groups) or Kruskal–Wallis test with Dunn's multiple comparisons (when we compare more than two groups). A p-value <0.05 is considered significant.
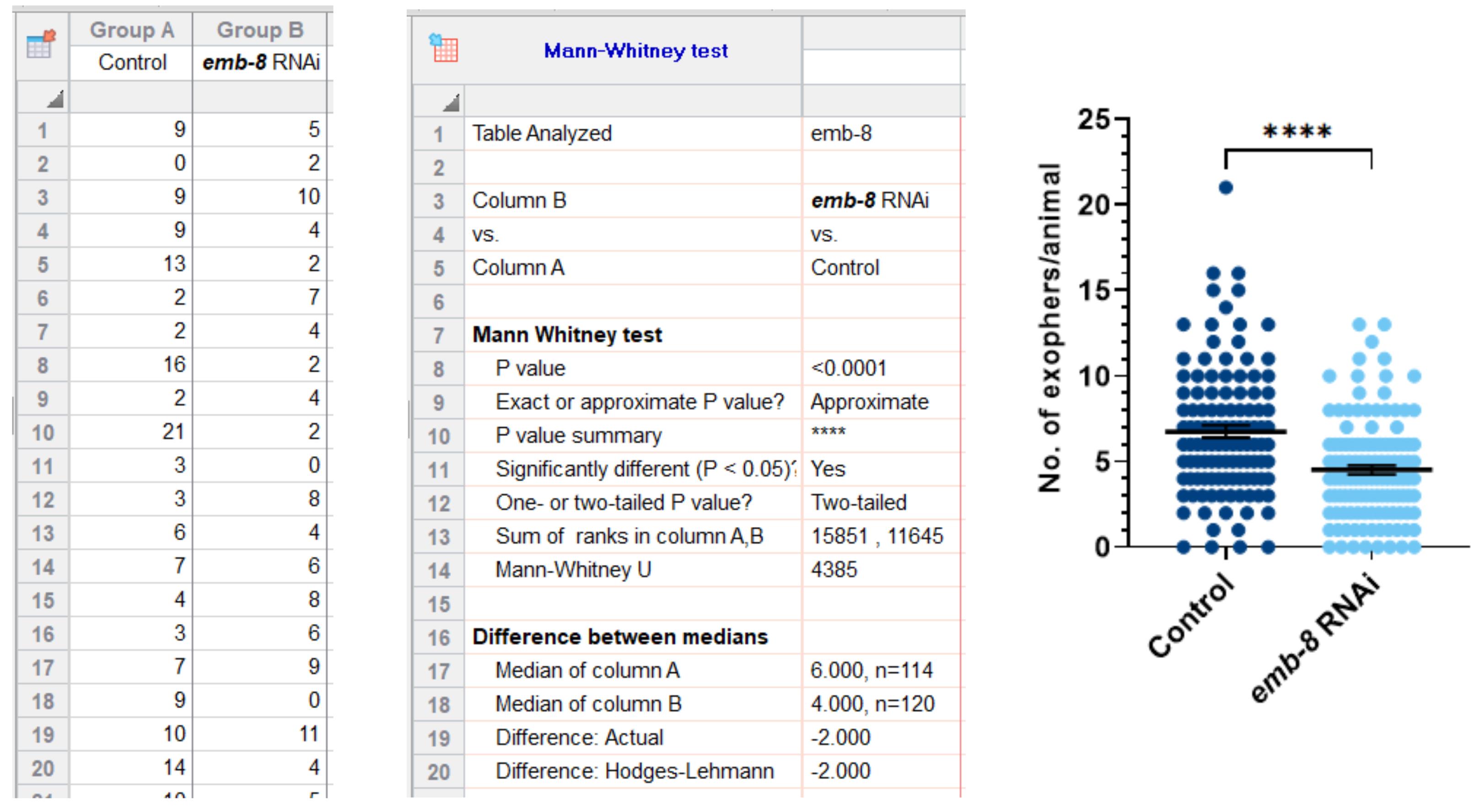
Figure 2. An example of an experiment subjected to quantitative, statistical analysis, and visualization in GraphPad Prism software. The formation of muscle-derived exophers is affected by the depletion of EMB-8 (emb-8 RNAi on strain ACH93). ACH93 worms fed on HT115 E. coli bacteria with the empty vector were used as control. **** P < 0.0001, Mann–Whitney test.
Notes
According to “Synchronous growth of larvae at later stages”, if worms are precisely synchronized at the beginning of the experiment, usually a maximum of 10%–20% of the worms need to be removed at the L4 stage. If more, be cautious at the initial steps of the procedure.
If scoring exophers in free-moving worms on a plate is too challenging initially, you may immobilize worms (with tetramisole) before you become proficient in counting them. The authors suggest removing freely moving hermaphrodites with already counted exophers directly afterward, to avoid unintentional re-measurement.
Recipes
Bacterial food source
Prepare a bottle of 500 mL LB (according to the manufacturer’s protocol).
Inoculate the LB in a flask with a single colony of OP50/HT115.
Incubate with shaking at 220 rpm at 37 °C overnight and, on the following day, seed the centrifuged 5× concentrated bacteria on NGM plates.
M9 buffer
Reagent Final concentration Na2HPO4 42 mM KH2PO4 22 mM NaCl 86 mM MgSO4 1 mM Nematode growth gedium (NGM) (for 750 mL)
Add 1.875 g peptone, 2.25 g NaCl, 12.75 g agar, and 712.5 mL of dH2O into a 1 L bottle and place a stir bar inside the bottle.
Autoclave for 20 minutes.
When the medium cools down to ~59 °C, add 7.5 mL of 0.1 M CaCl2 , 7.5 mL of 0.1 M MgSO4 , and 750 μL of filtered 5 mg/mL cholesterol. Stir with a magnetic stirrer for 3–5 min.
Add 18.75 mL of 1 M KPO4 , 750 μL of streptomycin, and 1.9 mL of nystatin. Mix for 1 min, then pour on plates with a peristaltic pump under sterile conditions next to the flame, or maintain at ~55 °C before pouring later that day.
Plates should be left to dry overnight, before seeding the worms.
Acknowledgments
ZEN (Zeiss), ImageJ (Fiji), and BioRender software were used to create the figures for this manuscript.
Funding: K. Banasiak and W. Pokrzywa’s work is funded by the Foundation for Polish Science and co-financed by the European Union under the European Regional Development Fund [grant POIR.04.04.00-00-5EAB/18-00]. M. Turek is supported by the National Science Centre SONATA-BIS grant [2021/42/E/NZ3/00358].
This protocol was developed for the study described in Turek et al. (2021).
Competing interests
The authors declare no competing interests.
References
- Altun, Z. F., and Hall, D. H. (2012). Introduction to C. elegans anatomy. WormAtlas.
- Arnold, M. L., Cooper, J., Grant, B. D. and Driscoll, M. (2020). Quantitative Approaches for Scoring in vivo Neuronal Aggregate and Organelle Extrusion in Large Exopher Vesicles in C. elegans. J Vis Exp (163). doi: 10.3791/61368.
- Gatica, D., Lahiri, V. and Klionsky, D. J. (2018). Cargo recognition and degradation by selective autophagy. Nat Cell Biol 20(3): 233-242.
- Melentijevic, I., Toth, M. L., Arnold, M. L., Guasp, R. J., Harinath, G., Nguyen, K. C., Taub, D., Parker, J. A., Neri, C., Gabel, C. V., et al. (2017). C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 542(7641): 367-371.
- Mizushima, N. and Komatsu, M. (2011). Autophagy: renovation of cells and tissues. Cell 147(4): 728-741.
- Mok, D. Z., Sternberg, P. W. and Inoue, T. (2015). Morphologically defined sub-stages of C. elegans vulval development in the fourth larval stage. BMC Dev Biol 15: 26.
- Nicolás-Ávila, J. A., Lechuga-Vieco, A. V., Esteban-Martinez, L., Sanchez-Diaz, M., Diaz-Garcia, E., Santiago, D. J., Rubio-Ponce, A., Li, J. L., Balachander, A., Quintana, J. A., et al. (2020). A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 183(1): 94-109 e123.
- Siddique, I., Di, J., Williams, C. K., Markovic, D., Vinters, H. V. and Bitan, G. (2021). Exophers are components of mammalian cell neurobiology in health and disease. bioRxiv: 2021.2012.2006.471479.
- Turek, M., Banasiak, K., Piechota, M., Shanmugam, N., Macias, M., Sliwinska, M. A., Niklewicz, M., Kowalski, K., Nowak, N., Chacinska, A., et al. (2021). Muscle-derived exophers promote reproductive fitness. EMBO Rep 22(8): e52071.
Article Information
Copyright
© 2023 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Banasiak, K., Turek, M. and Pokrzywa, W. (2023). Preparation of Caenorhabditis elegans for Scoring of Muscle-derived Exophers. Bio-protocol 13(1): e4586. DOI: 10.21769/BioProtoc.4586.
Category
Developmental Biology > Reproduction
Cell Biology > Cell imaging > Fluorescence
Molecular Biology > Protein > Protein shuttling
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








