- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Published: Vol 12, Iss 23, Dec 5, 2022 DOI: 10.21769/BioProtoc.4568 Views: 1601
Reviewed by: Edgar Soria-GomezNanci WinkeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
In situ Microinflammation Detection Using Gold Nanoclusters and a Tissue-clearing Method
Fayrouz Naim [...] Masaaki Murakami
Apr 5, 2023 2599 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2495 Views
A One-Step Mouse Model of Parkinson’s Disease Combining rAAV-α-Synuclein and Preformed Fibrils of α-Synuclein
Santhosh Kumar Subramanya [...] Poonam Thakur
Dec 5, 2025 1395 Views
Abstract
Pavlovian fear conditioning is a widely used procedure to assess learning and memory processes that has also been extensively used as a model of post-traumatic stress disorder (PTSD). Freezing, the absence of movement except for respiratory-related movements, is commonly used as a measure of fear response in non-human animals. However, this measure of fear responses can be affected by a different baseline of locomotor activity between groups and/or conditions. Moreover, fear conditioning procedures are usually restricted to a single conditioned stimulus (e.g., a tone cue, the context, etc.) and thus do not depict the complexity of real-life situations where traumatic memories are composed of a complex set of stimuli associated with the same aversive event. To overcome this issue, we use a conditioned lick suppression paradigm where water-deprived mice are presented with a single conditioned stimulus (CS, a tone cue or the context) previously paired with an unconditioned stimulus (US, a foot shock) while consuming water. We use the ratio of number of licks before and during the CS presentation as a fear measure, thereby neutralizing the potential effect of locomotor activity in fear responses. We further implemented the conditioned lick suppression ratio to assess the effect of cue competition using a compound of contextual and tone cue conditioned stimuli that were extinguished separately. This paradigm should prove useful in assessing potential therapeutics and/or behavioral therapies in PTSD, while neutralizing potential confounding effects between locomotor activity and fear responses on one side, and by considering potential cue-competition effects on the other side.
Graphical abstract

Schematic representation of the compound context-cue condition lick suppression procedure. Illustration reproduced from Bouchekioua et al. (2022).
Background
Pharmacological treatments for PTSD, such as the 5-HT2CR antagonist SB242084 (Browne et al., 2017), increase locomotor activity and could alternatively explain the reduction of fear responses when measuring the time spent in freezing. The potential locomotor activity confounding variable has thus to be neutralized before drawing any conclusion regarding the role of a treatment in fear responses.
Exposure therapies developed to treat PTSD consist of gradually confronting patients to stimuli that are identified as triggers of the symptoms; yet patients do not necessarily have conscious access to these triggers. Moreover, according to associative learning theories, the intensity of fear responses to stimuli can be affected in situations where multiple conditioned stimuli (CSs), previously paired with the same aversive event, interact (Kamin, 1969; Rescorla and Wagner, 1972; Mackintosh, 1974; Pearce and Hall, 1980). It has been shown that a reduction in fear responding to a cue is associated with sustained contextual fear responses when cue-specific and contextual information are combined during conditioning, but tested separately (Grillon et al., 2002; Baas et al., 2008; Baas, 2013). Underestimating cue-competition effects may thus lead to an incomplete evaluation of potential therapeutics.
To overcome these limitations and improve the screening efficiency of candidate drugs in fear conditioning, we propose a condition lick suppression procedure to assess contextual, cued, and context-cue compound fear responses.
Materials and Reagents
Black opaque acrylic or plastic sheet (height: 18 cm; width: 6 cm; thickness: 2 mm)
Triangular-shaped ceiling made with two transparent acrylic sheets (21.5 × 13 cm, 1 mm thick) (YSTIME, catalog number: B08NPMVJGQ)
Transparent acrylic hinges (IVYTOHO, catalog number: B07SMSJJK2)
0.5 mm thick high-density white polyethylene plastic sheet (Polymersan, catalog number: 0714038308181)
40 × 40 × 40 mm urethan rubber block (Tokyu Hands, catalog number: 4989606010889)
Solder wire (Editone, Adafruit, catalog number: SAC305)
Hook-up wire spool set, 22AWG solid core, 10 × 25 ft (Adafruit, catalog number: 3174)
Momentary push button switch NO NC AC 5 A/120 V (DIYhz, catalog number: B079D923V9)
DB9 D-SUB RS232 Adapter 9 pin signals terminal breakout plastic cover 2 row (male with screw) (Daier, catalog number: B01C2V2E8BU)
IR break beam sensors with premium wire header ends, 3 mm LEDs (Adafruit, catalog number: 2167)
MPR121 12 key capacitive touch sensor breakout (Adafruit, catalog number: B0778WYR5D) [Alternative: Adafruit 12 × capacitive touch shield for Arduino – MPR121 (Adafruit, catalog number: 2024)]
Adafruit LED Sequins [luminous intensity: minimum = 250 mcd and maximum = 350 mcd with IF (Forward Current) = 20 mA; luminous intensity measurement allowance is ± 10%], warm white (Adafruit, catalog number: 1758)
Speaker 8Ω8W (Akizukidenshi, catalog number: F00805)
Amazon Basics USB 2.0 printer cable – A-Male to B-Male cord (Amazon Basics, catalog number: B00NH11KIK)
White cotton/polyester knit gloves (Setaria Viridis, catalog number: B08QHLCZ11)
Double sided tape (3M, catalog number: 0888519024546)
Alligator to Dupont wire (Oiyagai, catalog number: B07CXLMBY7)
Adult mice (8-week-old to 18-week-old)
70% ethanol solution
Equipment
Modular chamber (Lab-hacks, model: STCHK0001)
Watering bottle with rubber stopper and water tube (CLEA Japan, catalog number: CL-0917)
Sound attenuating cubicle for mouse (Med associates, model: MED-OFA-022)
Standalone aversive stimulator/scrambler (115V/60Hz) (Med associates, model/catalog number: ENV-414S) [Alternative: Precision animal shocker (Coulbourne Instruments, Harvard Apparatus, catalog number: H13-15)]
Arduino Mega2560 Rev3 (Arduino, catalog number: A000067)
Raspberry Pi 3 model B+ [Raspberry Pi, catalog number: (Digi-Key)2648-RASPBERRYPI3MODELB+-ND]
Digital Genuine Hakko with a standard T18-D16 tip (Hakko, catalog number: FX-888D)
Software
Arduino IDE (Arduino, https://www.arduino.cc/en/software)
Anaconda (Anaconda, https://www.anaconda.com/)
Spyder IDE (Included in Anaconda, https://www.spyder-ide.org/)
Python 3 (https://www.python.org/downloads/)
Python libraries: pyserial 3.5 (https://pyserial.readthedocs.io/en/latest/pyserial.html); csv, time, and os modules are part of Python’s standard libraries
Excel (Microsoft, https://www.microsoft.com/fr-ww/microsoft-365/excel)
Prism 9 (GraphPad Software, https://www.graphpad.com/company/)
SPSS 23.0 (IBM, https://www.ibm.com/analytics/spss-statistics-software)
Arduino and Python scripts (https://github.com/YuYuB/Bio-Protocol-ConditionedLickSuppression)
Procedure
Handling and water deprivation
If mice are ordered from a supplier, group house same-gender mice (3–4 per cage) with unlimited access to food and water in a temperature/humidity-controlled (25 ± 2 °C, 40%–60%, respectively), 12 h reversed light/dark cycle room (light from 7 p.m. to 7 a.m.) for at least one week. If mice were born in the laboratory, make sure that they are group housed with same-gender animals (3–4 per cage), after being weaned at three weeks of age, with unlimited access to food and water in a temperature/humidity-controlled (25 ± 2 °C, 40%–60%, respectively), 12 h reversed light/dark cycle room (light from 7 p.m. to 7 a.m.). The whole procedure should be conducted during the dark phase of the light cycle (i.e., between 7 a.m. and 7 p.m.). Run mice in experiment 3 in context A during the morning and in context B during the afternoon. Run each mouse in experiments 1 and 2 within the dark cycle, around the same time of the first acclimation session (± 3 h).
Single house all mice and handle them for 5 min for at least three consecutive days during the dark cycle. To habituate a mouse to handling, pick it up from the tail with one hand, and place it on the other hand while wearing cotton/polyester knit gloves. Gently keep the tail on one hand while the mouse is standing or walking on the other hand to prevent escapes. Handling is considered complete when mice stop trying to jump out of the hand, after at least three consecutive days of handling.
The day following mice handling, remove water bottles from their home cage 24 h prior to the beginning of the experiment, during the dark cycle.
Allocate mice (N = 7–11) to one of eight groups (Figure 1):
Context-Only condition: Experimental group
Context-Only condition: No-treatment Control group
Context-Only condition: Unpaired Control group
Cued-Only condition: Experimental group
Cued-Only condition: No-treatment Control group
Cued-Only condition: Unpaired Control group
Compound condition: Experimental group
Compound condition: No-treatment Control group
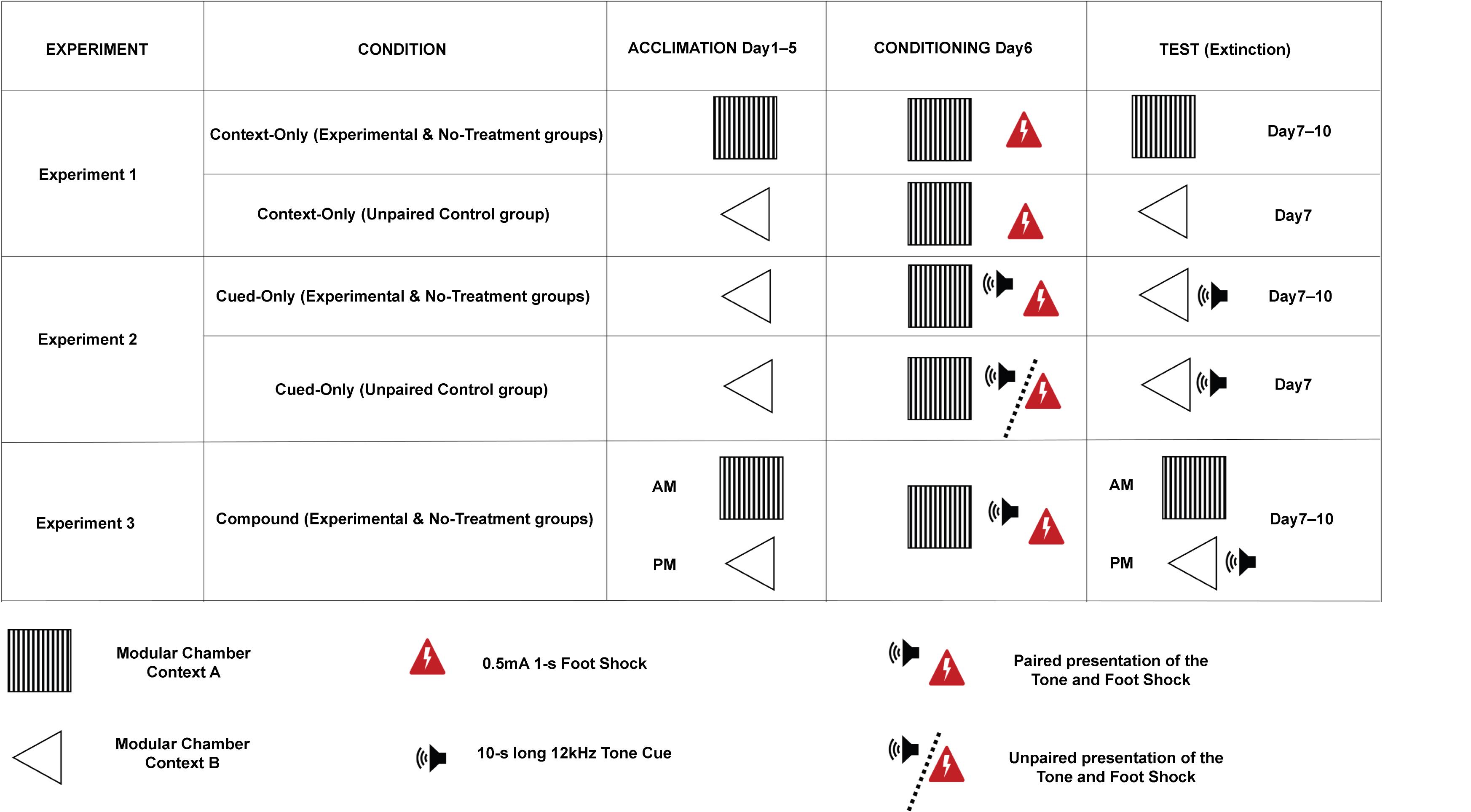
Figure 1. Schematic representation of the procedure. Experimental flowchart for each condition and group. The last three columns from the left represent the three different phases of the procedure (Acclimation, Conditioning, and Test). Illustration reproduced from Bouchekioua et al. (2022).Acclimation phase (Day 1 to Day 5)
Give each mouse a 30 min session of acclimation in the conditioning chamber inside a sound attenuating cubicle for five consecutive days (Figure 1). During the acclimation phase, mice have access to the water-filled lick tubes via the magazine. The number of licks and magazine entries are recorded. For the first two days of acclimation, leave two drops of water inside the magazine.
Clean the chambers between animals with a 70% ethanol solution during the whole experiment.
Make sure that the acclimation script “SPC_Acclimation.ino” (https://github.com/YuYuB/Bio-Protocol-ConditionedLickSuppression) is uploaded to the Arduino.
If connected, disconnect the USB cable from the Arduino to the USB port of your PC (Mac OS or Windows) or Raspberry Pi.
If you use a PC (Mac OS or Windows), open the Arduino IDE, left click Tools in the menu bar and place your mouse cursor over the Port row (Figure 2). Take note of the port names displayed, if any. Connect the Arduino USB cable to your PC and go back to the Port row of the Tools menu. Take note of the new port name that appears. It corresponds to the port name to which your Arduino is connected.
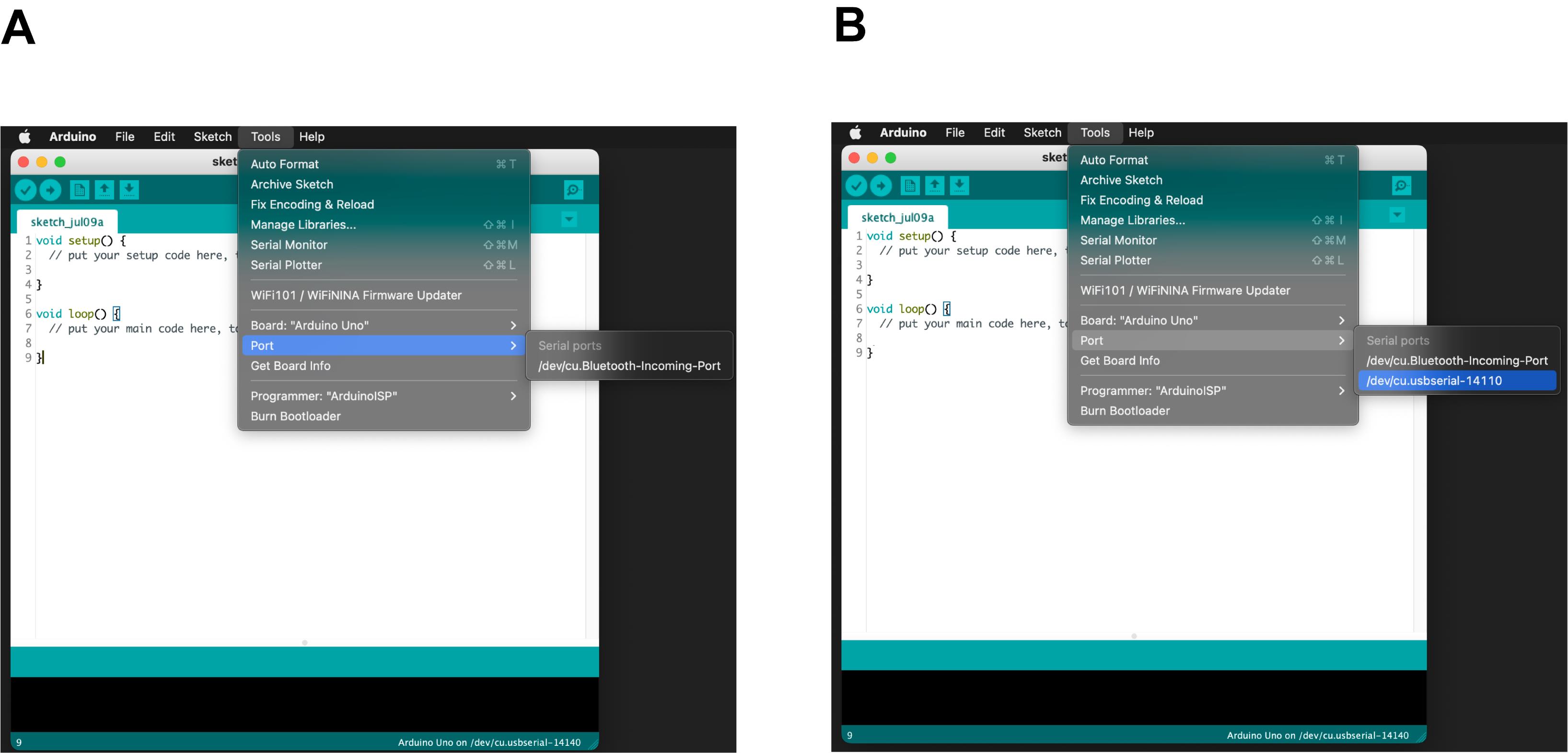
Figure 2. Display of the USB port name from the Arduino IDE. (A) List of port names before connecting the Arduino to the PC. (B) List of port names after connecting the Arduino to the PC, including the port name used by the Arduino (i.e., “/dev/cu.usbserial-14110” in this case).If you use a Raspberry Pi with Linux (or any PC with a Linux OS), open the command terminal, type “ls /dev/tty*”, and press the Enter key of your keyboard before connecting your Arduino to the USB port of the Raspberry Pi. Take note of the port names displayed. Connect the Arduino USB cable to your Raspberry Pi and repeat using the command “ls /dev/tty*” on your command terminal. Take note of the new port name that appears.
Open the Python 3 IDE (Spyder on PC and Python 3 IDLE on Raspberry Pi) and open the Python data collection script for the acclimation phase “Acclimation.py” (https://github.com/YuYuB/Bio-Protocol-ConditionedLickSuppression). Start running this Python script by pressing the Run File icon on Spyder (green right arrow) (Figure 3) or by left clicking Run in the menu bar and then left clicking on the Run File row on the Python3 IDLE of your Raspberry Pi. Alternatively, you can simply press the F5 key of your keyboard to start running the script.

Figure 3. Menu bar of Spyder. Screenshot of the menu bar of Spyder. The Run File icon is highlighted by a red circle.Type the PC/Raspberry Pi USB port name to which your Arduino is connected and press Enter.
Type a new file name and press Enter.
Finally, type the time of the session in seconds and press Enter.
Place the mouse in the conditioning chamber inside a sound attenuating cubicle and press the start button connected to your Arduino as soon as you release the mouse and close the front door of the chamber. Pressing the start button will turn the magazine line on. No other light is used for light housing.
Use Context A for mice of the Experimental and No-treatment Control groups of the Context-Only condition (Figure 4).
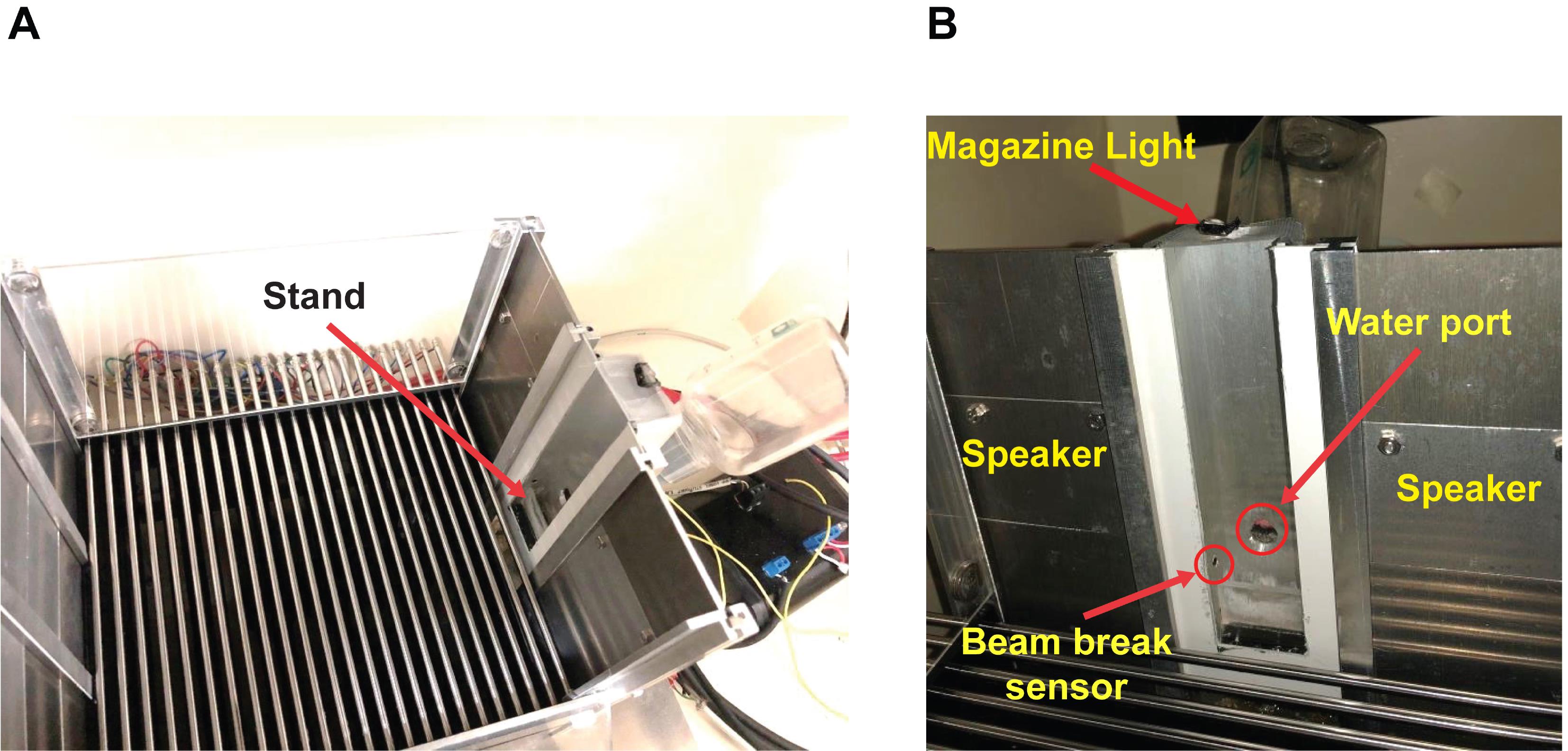
Figure 4. Context A during the acclimation phase. (A) Picture (from above) showing the configuration of the conditioning chamber used for Context A. The red arrow highlights a piece of transparent acrylic used as a stand and placed inside the magazine to facilitate access to the water port to mice. (B) Picture (from the inside of the chamber, facing the magazine) showing the configuration of the conditioning chamber used for Context A. The red arrows point to the magazine light, the two speakers, the water port, and the beam break sensor (the infrared light being at the opposite side of the magazine). The bottom and top of the speakers were at 3.5 and 8 cm from the grid floor, respectively. The speakers were fixed to the middle panel (i.e., the second panel from the top) of the wall (magazine side), using screws [cf., ChamberSideView (Water bottle and Speakers).jpeg, https://github.com/YuYuB/Bio-Protocol-ConditionedLickSuppression/tree/main/BioProtocol%20Github]. The bottom and top of the beam break LEDs were fixed with super glue and hot glue at 1.5 and 2 cm from the grid floor, respectively. The bottom and top of the water port were at 2.5 and 3 cm from the grid floor, respectively. Holes for the beam break LEDs and the water port were drilled to the aluminum magazine with a conventional drill and drill bits. The positions of the speakers, water port, and beam break LEDs were the same for both contexts (i.e., contexts A and B).Use Context B for mice of the Unpaired Control group of the Context-Only condition, and mice of the Experimental, No-treatment Control, and Unpaired Control groups of the Cued-Only condition (Figure 5).
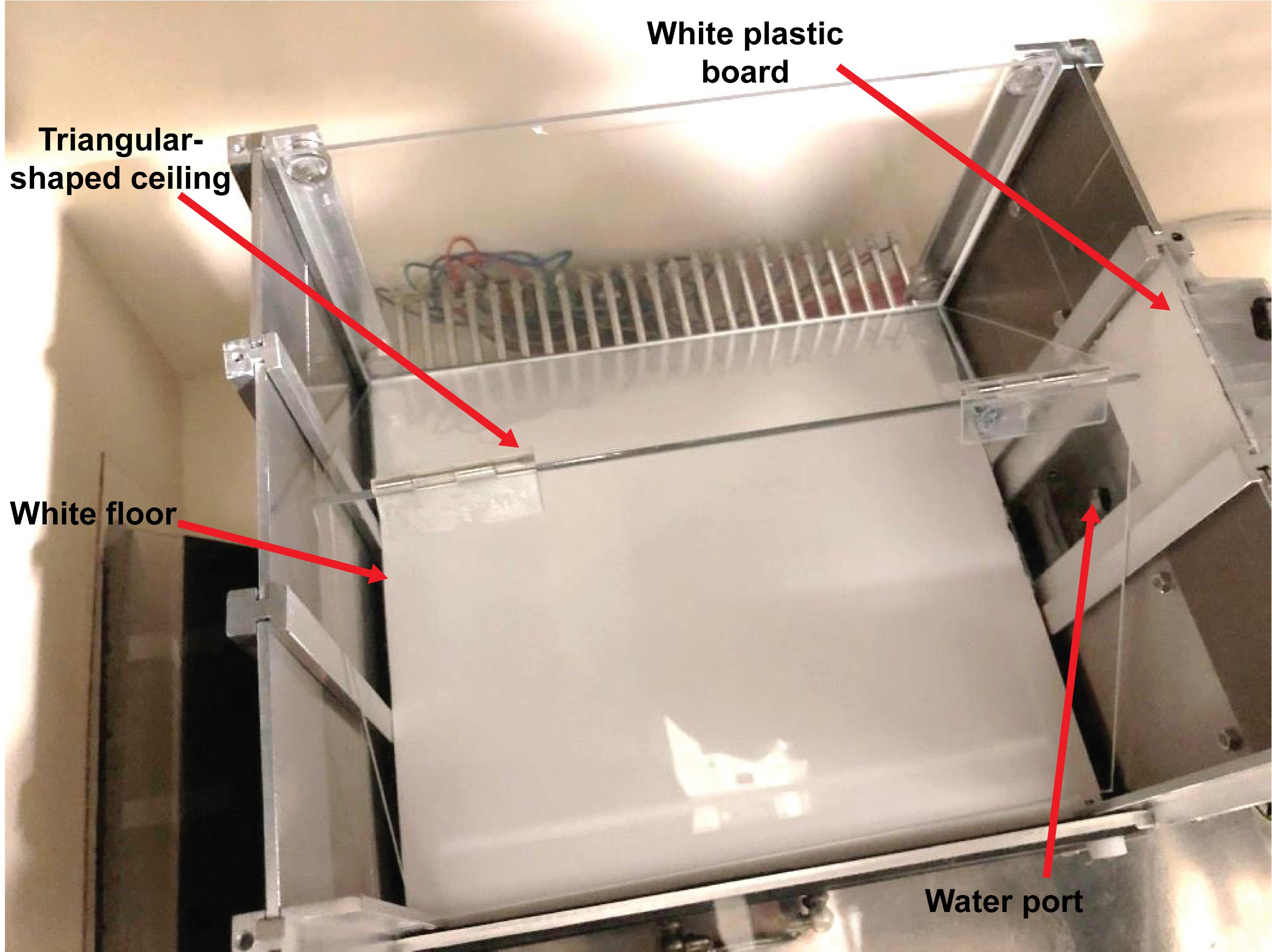
Figure 5. Context B. Picture (from above) showing the configuration of the conditioning chamber used for Context B. The red arrows highlight the white plastic floor, the triangular-shaped ceiling (made of two 1 mm thick acrylic boards connected with two transparent acrylic hinges and forming an angle of 80°), the water port, and a white plastic board used to prevent escapes to the top of the triangular-shaped ceiling.Run all mice of the Compound condition (i.e., Experimental and No-treatment Control groups) twice a day. Use Context A in the morning and Context B in the afternoon six hours later (Figure 1).
Remove the mouse from the chamber at the end of the 30 min session.
Give access to water for 30 min at home cage after each session to mice in conditions Context-Only and Cued-Only. Give access to water for 30 min at home cage after the last session of the day to mice in condition Compound.
Fear conditioning phase (Day 6)
Place a black opaque plastic/acrylic sheet in front of the magazine to prevent magazine entries and access to the water-filled lick tube (Figure 6).
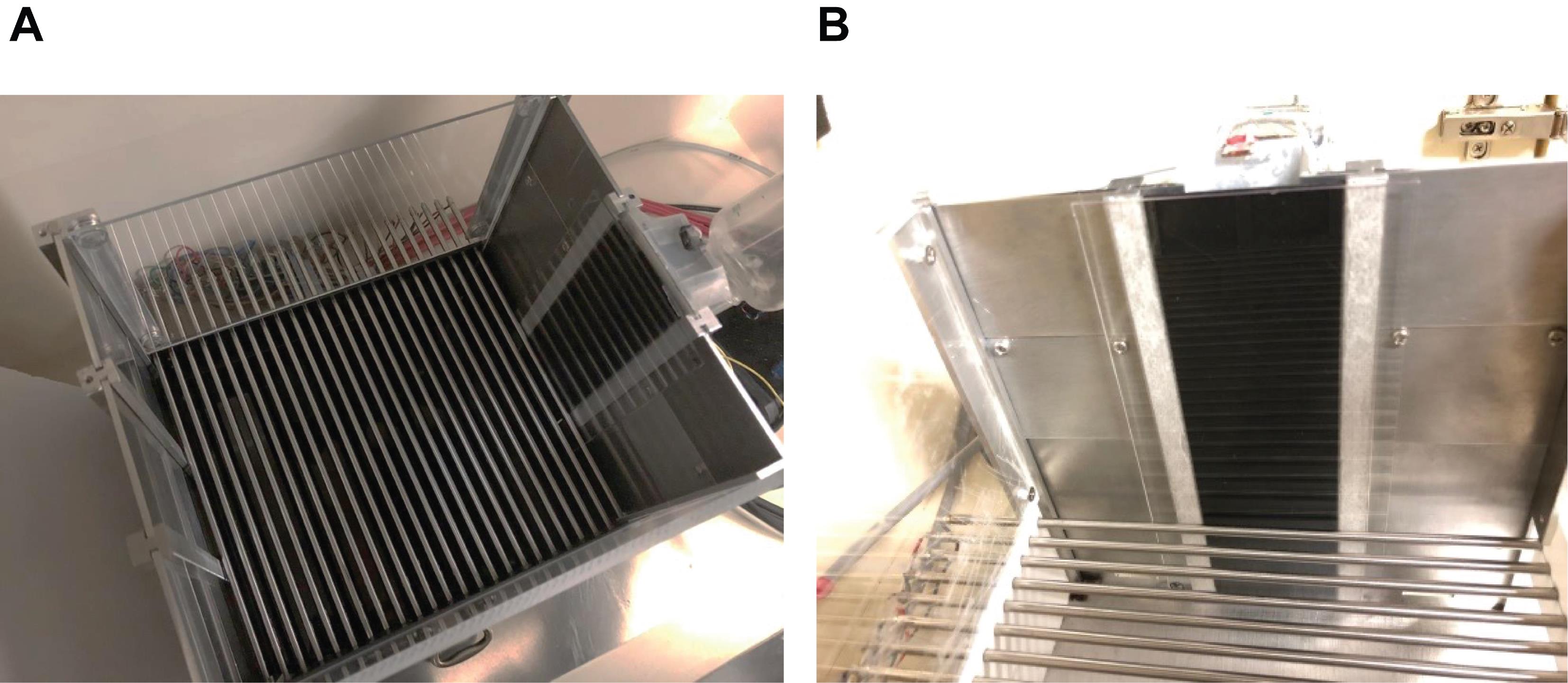
Figure 6. Context A during the conditioning phase. (A) Picture (from above) showing the configuration of the conditioning chamber used for Context A during the conditioning phase. The access to the magazine is prevented by adding an opaque plastic board in front of it. (B) Picture from the inside of the chamber, facing the magazine.Upload the Conditioning Arduino script (to the Arduino) that corresponds to the condition the mouse belongs to and make sure that the foot shocker is turned on, connected to the Arduino, and set up to 0.5 mA. Upload the script “SPC_Phase_2_CS-US_30min.ino” to the Arduino for groups Experimental and No-treatment of conditions Cued-Only and Compound, and upload the script “SPC_Phase_2_US_30min.ino” to the Arduino for groups Experimental and No-treatment of conditions Context-Only (https://github.com/YuYuB/Bio-Protocol-ConditionedLickSuppression). Upload the script “SPC_Phase_2_US-CS_30min.ino” to the Arduino for mice of the Unpaired group in condition Cued-Only (https://github.com/YuYuB/Bio-Protocol-ConditionedLickSuppression).
Mice of all groups and conditions, except the Unpaired group in condition Cued-Only, receive a 1 s, 0.5 mA foot shock at the following onsets: 5.15, 9.15, 14.15, 17.15, 21.15, and 25.15 min into the session.
Mice of the Unpaired group in condition Cued-Only receive a 1 s, 0.5 mA foot shock at the following onsets: 8.5, 11.7, 15.9, 18.3, 22.25, and 23.8 min into the session.
Mice of all groups in the Cued-Only and Compound conditions receive a 10 s, 12 kHz pure tone at the following onsets: 5, 9, 14, 17, 21, and 25 min into the session, that is, 9 s before the onset of each foot shock.
Connect the Arduino to the PC/Raspberry Pi.
Use Context A for mice of all groups and conditions (Figure 1).
Place the mouse in the conditioning chamber inside a sound attenuating cubicle and press the start button connected to your Arduino as soon as you release the mouse.
Remove the mouse from the chamber at the end of the 30 min session.
Give access to water for 30 min at home cage after each session to all mice.
Test phase (fear extinction: days 7–10)
Give each mouse a 30 min session of fear extinction in the conditioning chamber inside a sound attenuating cubicle for only one day for mice of the Control Unpaired groups and four consecutive days for mice of all the other groups (Figure 1). During the test phase, mice have access to the water-filled lick tubes via the magazine. The number of licks and magazine entries are recorded.
Follow steps B.1.a. to B.1.g except that the script for the Arduino and the Python data collection scripts should correspond to the test phase, condition, and groups.
Upload the script “Acclimation.ino” to the Arduino for all mice in condition Context-Only (https://github.com/YuYuB/Bio-Protocol-ConditionedLickSuppression) and use the Python script “Acclimation.py” on your PC/Raspberry Pi for data collection. Test sessions of all mice in condition Context-Only are the same as acclimation sessions.
Upload the script “SPC_TEST.ino” to the Arduino for all mice in condition Cued-Only and all mice in condition Compound for a.m. sessions (https://github.com/YuYuB/Bio-Protocol-ConditionedLickSuppression) and use the Python script “TEST.py” for data collection. Test sessions of all mice in condition Cued-Only and all mice in condition Compound for a.m. sessions consist of a tone cue delivery triggered up to five times by five cumulative seconds spent inside the magazine. The session ends if five tone cues are triggered, or after 30 min elapsed.
Test sessions of all mice in condition Compound are the same as the morning acclimation sessions (i.e., step C.2.a) and the Cued-Only test sessions in the afternoon (i.e., step C.2.b).
Data analysis
Inclusion criteria
Exclude mice that fail to drink from the water-filled lick tubes (i.e., 0 licks) on Days 1 and 2.
Data processing
Place the CSV files collected during the sessions you want to analyze into the same folder as the Python script for data processing corresponding to the condition and group you are analyzing. Use Python 3 for data processing. There are two Python scripts for data processing:
Use the Python script “Contextual fear extinction.py” (https://github.com/YuYuB/Bio-Protocol-ConditionedLickSuppression) to analyze data collected from all groups of condition Context-Only and from all groups of Compound in the morning session (i.e., for contextual fear extinction). Open the file generated by the Python script whose name has “bin” added to the original CSV file name. The number of licks during the 30 min session appears under the column entitled “Lick #”.
Use the other Python script “Cued fear extinction.py” (https://github.com/YuYuB/Bio-Protocol-ConditionedLickSuppression) to analyze data collected from all groups of condition Cued-Only and from all groups of Condition Compound in the afternoon session (i.e., for cued fear extinction). The suppression ratios for each tone triggered appear in the column entitled “Suppression ratio” of the CSV file generated by the Python script.
Conditioned lick suppression ratio
Baseline score
For all groups of condition Contextual-Only and Compound (a.m. sessions), calculate the baseline score by averaging the rates of licks during the 30 min sessions of the two last days of the acclimation phase (i.e., Days 4 and 5).
For all groups of condition Cued-Only and Compound (p.m. sessions), the baseline score is calculated by averaging the rates of licks during the 5 s period that precedes the onset of the tone cues.
Performance score
For all groups of conditions Contextual-Only and Compound (a.m. sessions), calculate the performance score by averaging the rates of licks during the 30 min sessions of each day of the test phase.
For all groups of conditions Cued-Only and Compound (p.m. sessions), the performance score is calculated by averaging the rates of licks during the first 5 s of the tone cues. The conditioned suppression ratio for each tone triggered was already calculated by the Python script and can be found in column “Suppression ratio” of the generated file whose name has “_analyzed” added to the original CSV file name. Calculate the averaged suppression ratio for each group.
Conditioned lick suppression ratio
For each group calculate the averaged conditioned lick suppression ratio as follows:
Conditioned Lick Suppression Ratio=(Baseline - Performance)/(Baseline + Performance)
For comparison between Experimental and Control Unpaired groups of the Cued-Only and Compound conditions, use the suppression ratio of the first triggered tone of Day 7 only (Figure 7).
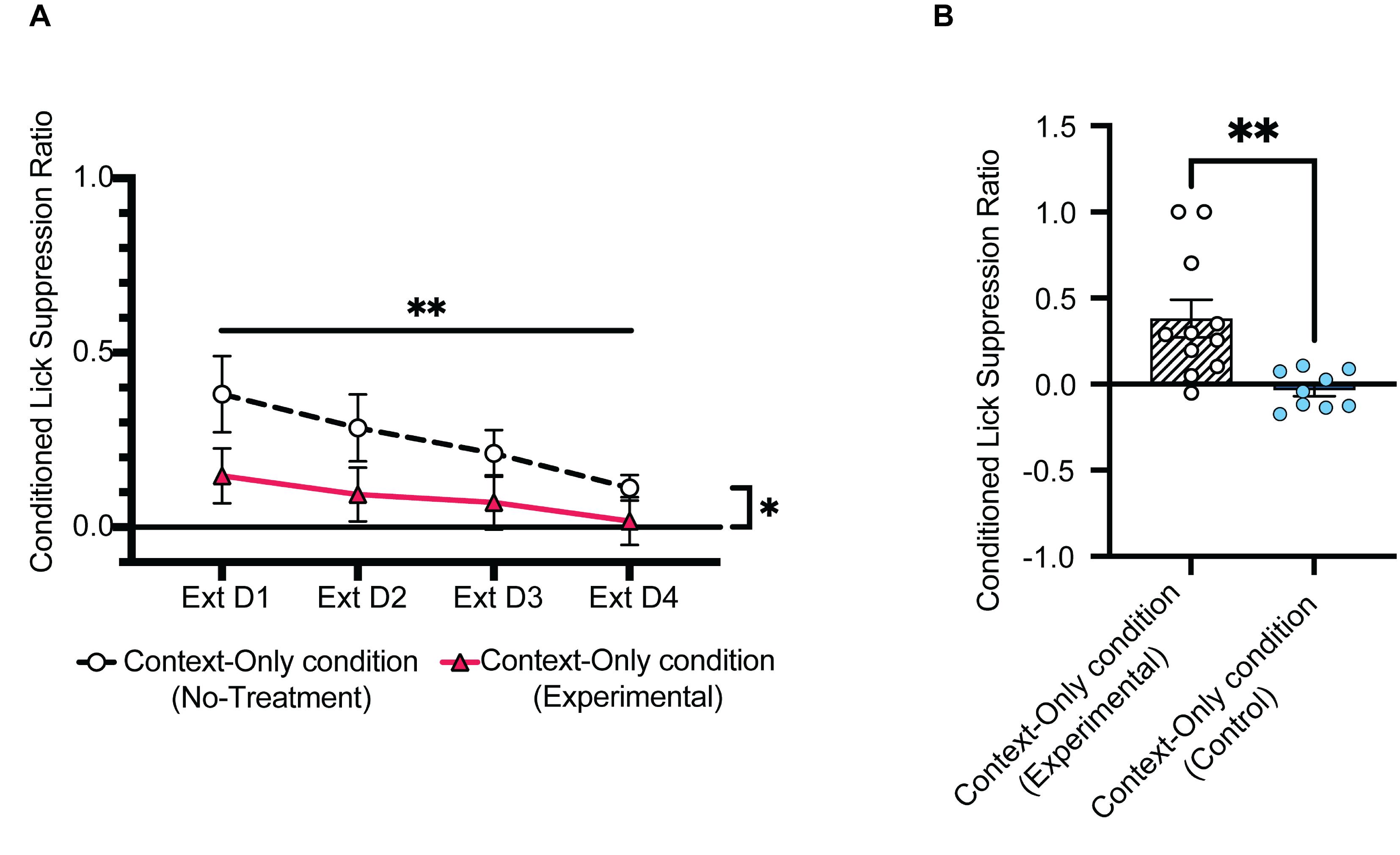
Figure 7. Representation of the lick suppression ratios in condition Context-Only. (A) Contextual lick suppression ratios (Context-Only condition; Days 7–10) of mice from the No-treatment group (C57BL/6N wildtype male mice; N = 11) compared to that of mice from the Experimental group [5-HT2CR KO male mice (RRID:IMSR_JAX:015821); N = 11]. (B) Contextual lick suppression ratios (Context-Only condition; Day 7, Tone 1) of mice from the Control group (C57BL/6N wildtype male mice; N = 9) compared to that of mice from the Experimental group [5-HT2CR KO male mice (RRID:IMSR_JAX:015821); N = 11]. Illustration reproduced from Bouchekioua et al. (2022).Survival rate (probability of survival)
For all groups of conditions Cued-Only and Compound (p.m. sessions), the survival rate corresponds to the number of tone cues triggered during a test session. The number of triggered tone cues appears in the column “Tone #” of the file generated by the Python script. The survival rate indicates the percentage of tone cues triggered within each group (Figure 8). A low number of tone cues triggered reflects a higher fear to the tone cue.
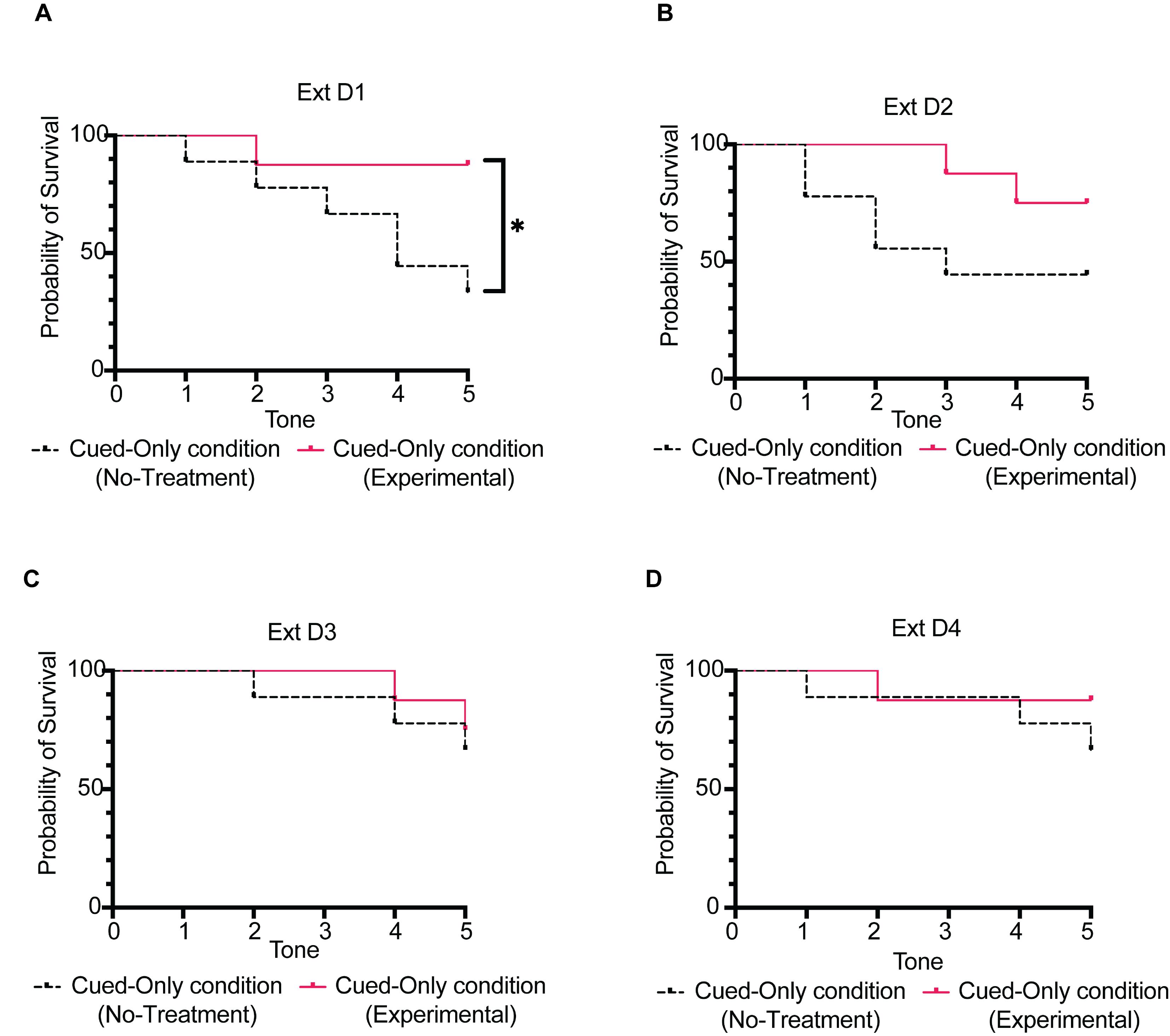
Figure 8. Representation of the survival rate in condition Cued-Only. Percentage probability of survival rate (Cued-Only condition; Days 7–10) of mice from the No-treatment group (C57BL/6N wildtype male mice; N = 9) compared to that of mice from the Experimental group [5-HT2CR KO male mice (RRID:IMSR_JAX:015821); N = 9]. (A) Cued fear extinction session at day 1 (Ext D1). (B) Cued fear extinction session at day 2 (Ext D2). (C) Cued fear extinction session at day 3 (Ext D3). (D) Cued fear extinction session at day 4 (Ext D4). Illustration reproduced from Bouchekioua et al. (2022).Statistical tests
Use an unpaired two-tailed Student’s t-test for independent samples, that is, for between-groups comparisons of lick suppression ratios during the first day of extinction (Day 7). As data from Experimental groups (Day 7) are used twice, use a Bonferroni correction.
Use the suppression ratio of Day 7 for comparison between Experimental and No-treatment groups of the Context-Only condition.
Use the suppression ratio of the first triggered tone of Day 7 only for comparison between Experimental and Control Unpaired groups, and between Experimental and No-treatment groups of the Cued-Only condition.
Use the suppression ratio of the first triggered tone of Day 7 only for comparison between Experimental and No-treatment groups of the Compound condition.
Use a two-way analysis of variance (ANOVA) for repeated measures, that is, for between-groups comparisons of lick suppression ratios across test sessions (fear extinction). Conduct a Bonferroni post-hoc test for multiple comparisons when applicable. If Mauchly’s sphericity test is significant, use Greenhouse-Geisser correction.
When comparing Experimental and No-treatment group of the Compound condition, use a mixed-effects ANOVA if not all mice trigger the same number of tone cues.
Use a log-rang (Mantel-Cox) test when comparing the survival rate performance between Experimental and No-treatment groups of the Cued-Only and Compound conditions.
Notes
Solder the wire connected to the MPR121 sensor directly to the water-filling lick tube (Figure 9).

Figure 9. Wire soldered to the water-filling tube. Picture showing the wire [connecting the capacitive touch sensor (MPR121) to the water-filling tube] soldered directly to the water-filling tube.Use a heat shrinking tube to isolate the water-filling lick tube and cover the wire that connects the MPR121 sensor to the tube (Figure 10). When mice drink from a non-isolated water-filling lick tube, the capacitive touch sensor will only count one lick if any body parts (e.g., nose) are in contact with the tube while they drink water. By isolating the tube, the capacitive touch sensor detects only contacts between the tongue and the ball inside the tube (that prevents water leaking). The heat shrinking tube also prevents the tube to be in direct contact with the magazine.

Figure 10. Heat shrinking tube. Picture showing the heat shrinking tube covering the water-filling tube and the wire connecting the capacitive touch sensor with the tube.To keep the water bottle in a fixed position with the spout of the water-filling tube being exposed inside the magazine, we super-glued a 40 × 40 × 40 mm urethane rubber block, previously drilled (10 mm of diameter) at an angle of approximately 45° [cf. “ChamberSideView (Water bottle and Speakers).jpeg”, available in our Github (https://github.com/YuYuB/Bio-Protocol-ConditionedLickSuppression/tree/main/BioProtocol%20Github]. Alternatively, we provide an *.STL file (“Lickometer MagAdapter.stl”) in the above-mentioned Github to 3D print a magazine adapter that supports the water bottle and includes a guide for the water-filling tube.
Connect the Arduino’s ground (one of the GND pin) to the modular chamber with an alligator to Dupont wire. We found that it prevents false capacitive touch signals.
Adapt the sensitivity of the sensor directly via its library (cf. “MPR121 Adafruit Doc” PDF file, p.15; https://github.com/YuYuB/Bio-Protocol-ConditionedLickSuppression).
Cover the top of the magazine with a plastic or acrylic board to prevent escapes from the conditioning chamber.
Use a white plastic board to cover the higher part of the magazine (cf. Figure 5) to prevent mice from escaping to the top of the triangular-shaped ceiling of context B.
Acknowledgments
This work was supported by JSPS KAKENHI grants (numbers: JP18K07545, JP19H04976, and JP21K07473) awarded to Y.O. and a JSPS KAKENHI grant (number: JP19K23377) awarded to Y.B. This protocol was derived from Bouchekioua et al. (2022). We thank Dr. Nebuka, Dr. Sasamori, Ms. Sugiura, Dr. Sato, and Prof. Yoshioka for their support in the study Bouchekioua et al. (2022), from which this current protocol was derived. We also thank Nick Normal, Head of QC Makerspace, for having provided access to 3D printers.
Competing interests
The authors declare no competing interests.
Ethics
The treatment of animals used in Bouchekioua et al. (2022) complied with the Guidelines for the Care and Use of Laboratory Animals of the Animal Research Committee of Hokkaido University and all procedures were approved by the Animal Research Committee of Hokkaido University (approval no. 18-0070).
References
- Baas, J. M., van Ooijen, L., Goudriaan, A. and Kenemans, J. L. (2008). Failure to condition to a cue is associated with sustained contextual fear. Acta Psychol (Amst) 127(3): 581-592.
- Baas, J. M. (2013). Individual differences in predicting aversive events and modulating contextual anxiety in a context and cue conditioning paradigm. Biol Psychol 92(1): 17-25.
- Bouchekioua, Y., Nebuka, M., Sasamori, H., Nishitani, N., Sugiura, C., Sato, M., Yoshioka, M. and Ohmura, Y. (2022). Serotonin 5-HT2C receptor knockout in mice attenuates fear responses in contextual or cued but not compound context-cue fear conditioning. Translational Psychiatry 12(1): 58.
- Browne, C. J., Ji, X., Higgins, G. A., Fletcher, P. J. and Harvey-Lewis, C. (2017). Pharmacological Modulation of 5-HT2C Receptor Activity Produces Bidirectional Changes in Locomotor Activity, Responding for a Conditioned Reinforcer, and Mesolimbic DA Release in C57BL/6 Mice. Neuropsychopharmacology 42(11): 2178-2187.
- Grillon, C. (2002). Associative learning deficits increase symptoms of anxiety in humans. Biol Psychiatry 51(11): 851-858.
- Kamin, L. J. (1969). In: Punishment and aversive behavior. (1st edition). Campbell, B. A. and Church, R. M. (Eds.). Ch. 9. Appleton-Century-Crofts, New York.
- Pearce, J. M. and Hall, l. G. (1980). A model for Pavlovian conditioning: Variations in the effectiveness of conditioned but not of unconditioned stimuli.Psychol Rev 87: 532-552.
- Mackintosh, N. J. (1974). The Psychology of Animal Learning. Academic Press, New York.
- Rescorla, R. A. and Wagner, A. R. (1972). In: Classical conditioning II: Current theory and research. Black, A. H. and Prokasy, W. F. (Eds.). Ch. 3. Appleton-Century-Crofts, New York.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Bouchekioua, Y., Nishitani, N. and Ohmura, Y. (2022). Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice. Bio-protocol 12(23): e4568. DOI: 10.21769/BioProtoc.4568.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Neuroscience > Nervous system disorders > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link