- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Modelling Graft-Versus-Host Disease in Mice Using Human Peripheral Blood Mononuclear Cells
(*contributed equally to this work) Published: Vol 12, Iss 23, Dec 5, 2022 DOI: 10.21769/BioProtoc.4566 Views: 3551
Reviewed by: Luis Alberto Sánchez VargasTakashi Nishina
Abstract
Graft-versus-host disease (GvHD) is a significant complication of allogeneic hematopoietic stem cell transplantation. In order to develop new therapeutic approaches, there is a need to recapitulate GvHD effects in pre-clinical, in vivo systems, such as mouse and humanized mouse models. In humanized mouse models of GvHD, mice are reconstituted with human immune cells, which become activated by xenogeneic (xeno) stimuli, causing a multi-system disorder known as xenoGvHD. Testing the ability of new therapies to prevent or delay the development of xenoGvHD is often used as pre-clinical, proof-of-concept data, creating the need for standardized methodology to induce, monitor, and report xenoGvHD. Here, we describe detailed methods for how to induce xenoGvHD by injecting human peripheral blood mononuclear cells into immunodeficient NOD SCID gamma mice. We provide comprehensive details on methods for human T cell preparation and injection, mouse monitoring, data collection, interpretation, and reporting. Additionally, we provide an example of the potential utility of the xenoGvHD model to assess the biological activity of a regulatory T-cell therapy. Use of this protocol will allow better standardization of this model and comparison of datasets across different studies.
Graphical abstract

Background
Hematopoietic stem cell transplantation (HSCT) is a therapeutic strategy used to treat certain immune system disorders as well as various hematological cancers such as myeloma and leukemia (Gschweng et al., 2014). In this procedure, recipients are typically conditioned with irradiation and/or chemotherapy to deplete immune cells, which are, in turn, replaced by an intravenous infusion of hematopoietic stem cells. As the hematopoietic stem cells are often derived from an allogeneic donor, a severe and potentially lethal side effect of HSCT is graft-versus-host disease (GvHD), whereby the patient’s new immune cells recognize the recipient’s cells as foreign and attack and destroy healthy tissues (Ferrara et al., 2009). This process is thought to be driven by reconstituted T cells recognizing allogeneic human leukocyte antigens (HLAs) on recipient cells (Loiseau et al., 2007).
Animal models are critical for testing novel therapeutic strategies that can be used to treat GvHD. Mouse models of GvHD are well established and have significantly advanced this field of research. However, in order to examine how new therapies influence the function of human T cells, models in which mice are reconstituted with human immune cells (humanized mice) are required. Accordingly, significant work has been performed to generate immunodeficient mice that can be reconstituted with human cells from different sources. For example, NOD-scid-IL-2Rγ null (NSG) mice are immunodeficient for B, T, and natural killer (NK) cells and have defective dendritic cells and macrophages, allowing them to be efficiently engrafted with human cells/tissues (Ehx et al., 2018). However, each humanized mouse model has unique properties that must be carefully considered when attempting to answer specific scientific questions. For example, it is well established that following peripheral blood mononuclear cell (PBMC) reconstitution of NSG mice, the primary cells to engraft are T cells, making this model unsuitable for assessing humoral immune responses. Additional factors such as time and cost should also be considered when selecting an appropriate humanized mouse model. This area has been extensively reviewed in Walsh et al. (2017), Adigbli et al. (2020), and Khosravi-Maharlooei et al. (2022).
For modelling the multi-system disorder known as xenoGvHD, we and others have shown that human T cells readily engraft in NSG mice upon intravenous infusion. These human T cells are stimulated in a xenogeneic manner via the recognition of mouse major histocompatibility complex molecules, and as such, the consequential pathology observed in these mice closely mimics human GvHD (King et al., 2009). Although PBMC-reconstituted xenoGvHD mouse models have their limitations, such as the lack of a complete human immune system, they can be used to generate safety data and conduct pre-clinical proof-of-concept data for T cell–directed therapies.
This protocol describes a method for inducing and monitoring xenoGvHD in NSG mice. We provide details on human PBMC preparation, methods to monitor disease progression over time, and an approach to data analysis and reporting. We also provide an example of how this model can be used to demonstrate the therapeutic potential of regulatory T cells (Tregs).
Materials and Reagents
Materials
Sterile 1.5 mL microcentrifuge tubes (Fisher Scientific, catalog number: 229442)
15 mL Falcon tubes (Corning, catalog number: 14-959-53A)
50 mL Falcon tubes (Corning, catalog number: 14-432-22)
Heparinized capillaries (Fisherbrand, catalog number: 22-362-566)
2” × 2” gauze (Fisherbrand, catalog number: 22-362178)
Cotton tipped applicators (Q-tips®)
Petroleum jelly (i.e., Vaseline®)
27 G needles, one per animal (BD Biosciences, catalog number: 305109)
Appropriate mouse restrainer for blood collection
70 µm cell strainer (Corning, catalog number: 352350)
96-well clear V-Bottom polystyrene not treated microplate (Corning, catalog number: 3896)
0.5 mL insulin syringes (BD Horizon, catalog number: 329461)
1 mL syringe (BD Horizon, catalog number: 309659)
Weighing bucket (empty 1 mL tip box without the rack or any small box)
Micro test tubes (Bio-Rad, catalog number: 2239391)
Corning® cryogenic vials, internal thread (Corning, catalog number: CLS430488)
Mice
8–16-week-old NOD/SCID/IL-2Rgammanull (NSG) mice (The Jackson Laboratory, Strain: 005557)
Note: Select the sex of mice depending on the experimental question. Both commercially available and house bred mice may be used. We have used mice of either sex and have not observed any differences in the induction of xenoGvHD.
Drugs
Anesthetic, isoflurane (USP 250 mL) (Piramal Critical Care Inc, part number: DVM-102190)
Reagents
Heparin (Sigma, catalog number: H3149-10KU)
70% ethanol (VWR, catalog number: 89370-078)
Fixation/permeabilization concentrate (eBioscience, catalog number: 00-5123-43)
Fixation/permeabilization diluent (eBioscience, catalog number: 00-5223-56)
10× permeabilization buffer (eBioscience, catalog number: 00-8333-56)
Counting beads (123count eBeadsTM, Invitrogen, catalog number: 01-1234-42)
DNase I solution (1 mg/mL) (StemcellTM Technologies, catalog number: 07900)
Penicillin–streptomycin (P/S) (GibcoTM, catalog number: 15140122)
β-mercaptoethanol (Sigma-Aldrich, catalog number: M3148)
Dithiothreitol (DTT) (ThermoFisher, catalog number: 20290)
EDTA (Sigma-Aldrich, catalog number: 03690)
Collagenase from Clostridium histolyticum (Sigma-Aldrich, catalog number: C7657)
Percoll (Sigma-Aldrich, catalog number: P1644)
Media and Buffers
10× RBC lysis buffer (eBioscience, catalog number: 00-4300-54)
Gibco 1× Dulbecco’s phosphate buffered saline (DPBS) (ThermoFisher, catalog number: 14190)
Gibco 10× Phosphate buffered saline (PBS) (ThermoFisher, catalog number: 70013032)
LymphoprepTM (StemcellTM Technologies, catalog number: 07851)
Immunocult-XF T-cell expansion medium (StemcellTM Technologies, catalog number: 10981)
Gibco fetal bovine serum qualified (FBS) (ThermoFisher, catalog number: 12483020)
Gibco RPMI 1640 medium (ThermoFisher, catalog number: 11875093)
Gibco GlutaMax (ThermoFisher, catalog number: 35050061)
Gibco HEPES (1 M) (ThermoFisher, catalog number: 15630080)
Antibodies
Mouse Fc block (BD Biosciences, catalog number: 553142)
Fixable viability dye (FVD) eFluor 780 (eBioscience, catalog number: 65-0865-18)
Anti-Mouse CD45 (30-F11) AF700 (BD Biosciences, catalog number: 560510)
Anti-Human CD45 (HI30) V500 (BD Biosciences, catalog number: 560777)
Anti-Human CD4 (SK3) BV786 (BD Biosciences, catalog number: 563877)
Anti-Human CD8 (RPA-T8) BV711 (BD Biosciences, catalog number: 563677)
Anti-Human CD3 (UCHT1) BB515 (BD Biosciences, catalog number: 564466)
Anti-Human Foxp3 (236A/E7) PE-Cy7 (eBioscience, catalog number: 25-4777-42)
Anti-Human Helios (22F6) AF488 (BioLegend, catalog number: 137223)
Anti-Human HLA-A2 (BB7.2) APC (Invitrogen, catalog number: 17-9876-42)
Equipment
Type II biosafety cabinet (NuAire, model: LabGard ES NU-540)
Microcentrifuge (Eppendorf, models: 5810R and 5452)
Cell counter (e.g., Nexcelom, model: Cellometer Auto 2000 or alternative)
X-ray irradiator (Rad Source-RS2000 Pro Biological Irradiator; alternate instruments including a gamma irradiator can be used)
Dosimeter (Radcal-2186 Dose Meter; alternative instruments can be used)
Electric shaver (Wahl Professional Animal BravMini+ Pet Trimmer, catalog number: 41590-0437)
Ear notcher (Fine Science Tools, catalog number: 24214-02)
Flow cytometer (BD LSRFortessaTM X-20; alternative instruments can be used)
OHAUSTM NavigatorTM portable balance (Thermo Fisher, catalog number: 01-922-205)
Thermo ScientificTM PrecisionTM general purpose baths (Thermo Fisher, catalog number: TSGP05)
VetFloTM vaporizer single-channel anesthesia system (Kent Scientific Corporation, catalog number: VetFlo-1205SP)
Software
FlowJo (BD Biosciences, 10.8.1)
Procedure
Experimental Timeline
Day -1: Prepare mice
Day 0: Inject PBMCs
Day 2 onwards: Monitor until endpoint
Day 7 onwards: Weekly bleeding to monitor engraftment
Detailed procedure
Day -1: Prepare mice for infusion of PBMCs
Create experimental groups
Ear notch mice for identification.
Randomly allocate mice to experimental groups. Mice should be housed under sterile conditions according to institutional guidelines and local ethical approvals. For reference, we house 2–4 mice per cage (UBC Animal Care Committee approval A18-0180). For the best practice, randomize mice and group them in a blinded fashion. Allocate littermates of the same cage to different experimental groups to control for cage-to-cage variation.
Note: Tail marking may be used instead of ear notch, but this is short-lasting and requires repeated application.
Weigh mice
Wipe a weighing bucket with 70% ethanol and place on a portable balance in a biosafety cabinet.
Gently transfer a mouse to the weighing bucket and record the weight.
Wipe the weighing bucket and gloves with 70% ethanol between cages.
OPTIONAL: Irradiate mice
Keeping the mice in their cage, irradiate with 150 cGy (150 rad) X-ray irradiation (exposure time to obtain 150 cGy varies between machines and should be established in consultation with local animal facility staff). Confirm the total accumulated dose using a dosimeter.
Note: Preconditioning mice with X-ray irradiation is optional. The recommended dose is sublethal and considered as mild (Gibson et al., 2015). Irradiation facilitates faster and more consistent engraftment and significantly accelerates the onset of xenoGvHD (King et al., 2009).
Monitor mice
Check mice for signs of stress/discomfort immediately after irradiation and 4 h later. Examples of such signs are provided in Table 1. If signs of stress/discomfort are observed, consult the veterinarian of your animal facility or euthanize the animal.
Irradiation may cause minor (<10%) weight loss, which should be regained within five days. If the weight loss continues, humanely euthanize the animal and remove from the study.
Table 1. Animal monitoring sheet with xenoGvHD scoring guide
xenoGvHD score guide 0 1 2 3 Weight loss (%) <5% 5%–10% 10%–15% >15% Activity Bright and alert; interested in the environment; interacts with casemates; looks at observer; when nudged, moves away or sniffs observer. Less interested in the environment; interacts less with casemates; disregards observer; when nudged, reluctantly moves away or hyperactive. Isolated from casemates; sits in corner of cage; does not readily move when cage is disturbed (only moves when touched); when nudged, pauses before moving away slowly. Immobile or hyperreactive, even when nudged; cannot right itself. Hunch Normal posture. Mild or occasional hunching (round). Moderate hunched posture (easily noticed). Severe hunched posture (tip toe gait). Fur/skin Shiny, well-kept coat. Unkempt, dull, or soiled coat, ruffled fur;skin is mildly inflamed (mildly red).Fur loss (may be generalized with skin exposed by caressing or localized and presenting as alopecia—especially on face, neck, hinds);skin is moderately inflamed or affects face or footpads.Fur loss on 25% of mouse, soiled dull fur;skin is ulcerated (face, footpads). Note: conjunctivitis is also a sign of GvHD (1 = discharge, pus; 2 = closing, whitening of cornea).Pain n/a n/a Self-trauma; facial grimace: narrow or closed eyes, bulge on top of nose (mice), flattening of bridge of nose (rat), cheek convex (mice: between eye and whiskers), ears back or flat, whiskers pointing back or “standing out on end”. Other: muscle twitching or flinching, staggering, back stretch (like cat), abdominal writhing, abdominal pressing. Nothing additional, monitor as per protocol Nothing additional, monitor as per protocol Monitor mice every day Euthanize immediately Animals with a score of 2 in any one category: score every day.Experimental endpoints: cumulative score of six or seven weeks after injection of cells.Humane endpoints: score 3 in any one category.Other endpoints: Mice showing any signs of infection or severe condition such as eyelids closed, changes in respiration, loss of fur, unusually apprehensive or aggressive, scratching, biting or self-mutilation, hunched posture, aggressive vocalization, separation from group, or sunken or distended abdomen will be euthanized.
Day 0: Preparation and injection of PBMCs
Prepare PBMCs in a biosafety cabinet: either freshly isolated or thawed PBMCs may be used.
Fresh cells: Isolate PBMCs from human peripheral blood using LymphoprepTM, according to established protocols (https://protocols.io/view/can-asc-consensus-protocol-isolation-cryopreservat-brrsm56e.pdf). PBMCs can be infused immediately after isolation or stored at 4 °C overnight and infused the following day. For the latter, cells should be stored in a 50:50 mixture of PBS and Immunocult-XF at a concentration of 10 × 106 cells/mL in a 50 mL Falcon tube laid horizontally.
Frozen cells: Frozen PBMCs can be thawed on the day of infusion. Using batch frozen PBMCs allows the cells to be characterized before experimentation and can reduce experimental variability. An example of the variability that can result from using PBMCs from different donors is shown in Figure 1. An intravenous infusion of 5 × 106–10 × 106 PBMCs should achieve an engraftment level of >20% human CD45+ cells in the peripheral blood 14 days post infusion and xenoGvHD symptoms should be evident within 3–4 weeks. Using PBMCs that do not achieve these criteria may yield sub-optimal results.
Note: Number of PBMCs may be a limiting factor. For large experiments, commercial leukopaks can be used as a frozen source of PBMCs.
Prepare Immunocult-XF T-cell expansion media with 1% P/S. Warm up the media to 37 °C in a water bath. Add 10 mL of the media to 15 mL Falcon tubes. Prepare one 15 mL Falcon tube for each cryogenic vial.
Note: We use Immunocult-XF T-cell expansion media to avoid the use of human serum, which often shows batch variations in composition. Any other T-cell expansion medium can be used here for the thawing process.
Thaw cryovials in a 37 °C water bath until almost completely thawed.
Add 1 mL of warm thawing media dropwise to cryotubes, then carefully transfer cells to a 15 mL Falcon tube containing 9 mL of warm media.
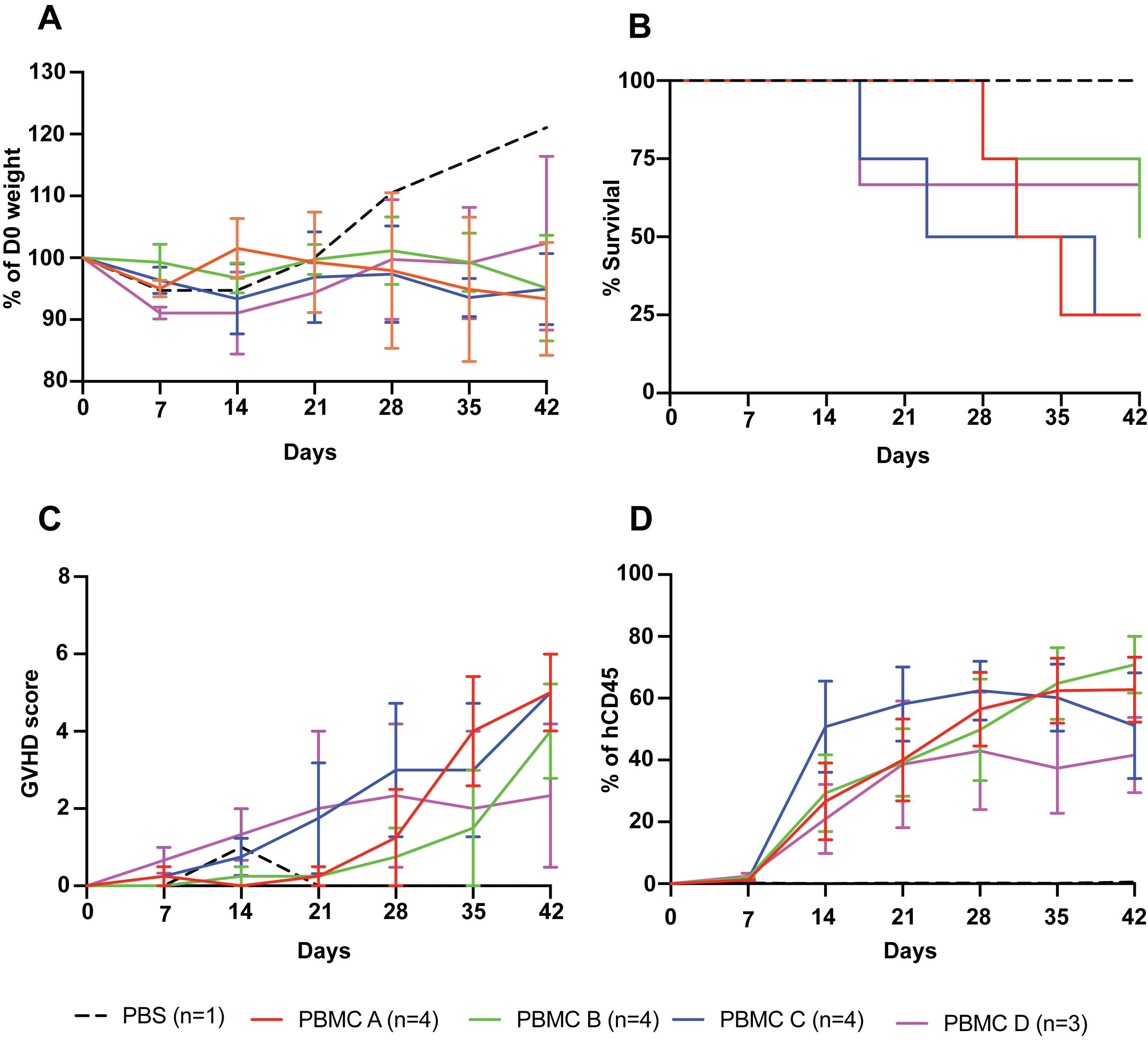
Figure 1. PBMC donor variation in inducing xenoGvHD. Five million PBMCs from four different donors (PBMC A: male; PBMC B: female; PBMC C: male, and PBMC D: female) were tested for their ability to induce xenoGvHD. (A) % weight loss relative to day of PBMC infusion (day 0). (B) % mouse survival. (C) Cumulative GvHD score. (D) Engraftment of human (h) CD45+ cells as a proportion of total live CD45+ (mouse and human) cells.Note: For mice that were terminated before the endpoint (D42), the end date value for the calculation was carried forward in subsequent time points.
Centrifuge cells at 450 × g for 5 min and discard the supernatant. To reduce cell clumping, resuspend the cells in 10 mL of Immunocult-XF + 1% P/S with DNase I at a concentration rate of 10 µg/mL, gently mix by pipetting, take a sample for counting, and incubate for 10 min at room temperature.
Centrifuge cells at 450 × g for 5 min, discard the supernatant, and resuspend the PBMCs in sterile PBS at a concentration of > 100 × 106 cells/mL (approximately 120 × 106/mL).
Pass cells through a 70 µm cell strainer, recount, and adjust the cell concentration to 100 × 106 cells/mL.
Note: The cell concentration adjusted here is for a dose of 10 × 106 PBMCs in 100 µL injection volume. If you intend to inject a different number of cells, adjust the cell number such that 100 µL of PBS contains the desired number of cells. The number of cells required for inducing xenoGvHD varies between PBMC donors, thus a pilot study to assess the engraftment capacity of cryopreserved PBMC aliquots is strongly recommended. If additional agents or cells of interest (e.g. Tregs) are co-administered with the PBMCs, the final infusion volume should not exceed 1% of the mouse body weight (i.e., 200 µL for a 20 g mouse). Follow guidelines from your animal ethical committee for the maximum injection volume via tail vein.
Just before cell injection, transfer cells to a 1.5 mL tube and store on ice until injection to minimize the time between cell storage on ice and cell injection.
Note: On the day of injection the viability of PBMC should be higher than 70%.
PBMC injection: Remove cells from ice 10–15 min before injection to warm up the cell suspension to room temperature. Gently mix the PBMCs by inverting the 1.5 mL collection tube. Draw 100 mL of cells into an insulin syringe and ensure no bubbles are present. Inject directly in the tail vein with the bevel pointing upwards, following the method below:
Notes:
1) The temperature of circulatory blood in the mouse tail vein is approximately 37 °C. Warming up cells to 37 °C or room temperature before adoptive transfer minimizes the heat shock to cells and ensures better survival in vivo.
2) Limit drawing the cells up and down into the syringe as much as possible; instead, mix by inversion. Repeatedly drawing the cells in and out of the syringe exposes them to unnecessary sheer stress that reduces viability. If you are injecting any other cells with the PBMC, cells can be mixed together and injected as a single inoculation in a total volume of 200 µL.
Vasodilate the tail vein of mice prior to intravenous injections to help with visualization. This can be performed using one of the following approaches:
Place half of the cage on top of a safe heat source such as a heat pad. Only half of the cage should be on the heat source so that there is a cooler half of the cage where mice can move to in case of overheating.
Apply heat directly to the tail itself using a glove filled with lukewarm water. Make sure the water is not too hot to avoid burn injury.
Any of the above methods can be used for efficient vasodilation, but never leave mice unattended around heating sources.
Place mice in a restrainer if injecting conscious, or in a warmed anesthetizing induction chamber if injecting anesthetized mice.
Note: If injecting an anesthetized mouse, make sure to inject as quickly as possible since the anesthetic agents can cause vasoconstriction making it difficult to find the vein.
Hold the warmed tail with the non-dominant hand and locate one of the two lateral tail veins. Swab the tail with 70% ethanol (avoid applying too much alcohol, which may cause vasoconstriction) to disinfect and increase the visibility of the vein. Then, insert the needle bevel up into the distal portion of the middle third of the tail with the dominant hand.
Slowly press the plunger to test if you are in the vein. If the needle is in the vein there will be no resistance; however, you will feel resistance if it is not in the vein. Once confident that you are in the vein, gently inject 100 µL of cell suspension. Keep the needle in place for 2–3 s to limit backflow of the injected cells, then remove the needle and press the injection site with a sterile gauze to stop bleeding and limit backflow of cell suspension. Monitor the animals for 5–10 min for any sign of discomfort or bleeding.
Note: While injecting cells in the tail vein do not pull the plunger back, as this may cause clumping of cells. We recommend using insulin syringes for injections as there is less dead space, which minimizes volume loss during injection. See Yano et al. (2020) for a detailed video protocol.
Day 2 to endpoint: Monitoring
Monitor body weight and health status of mice according to the approved animal use protocol. For our protocol (UBC Animal Care Committee approval A18-0180), this is three times per week.
Bleed mice by puncturing the saphenous vein once every seven days post cell injection to monitor cell engraftment.
Euthanize mice and end experiment according to approved animal protocol. In our case, this is when any of the following criteria are achieved: i) >15% body weight loss, ii) any health score category is ≥3, or iii) the cumulative health score is ≥6 (Table 1).
Collect blood from the lateral or medial saphenous veins following the steps below:
Prepare blood collection tubes (1.5 mL Eppendorf) by adding 3 µL of heparin (50 mg/mL) into separate tubes for each animal.
Place a mouse in an appropriate restrainer with your non-dominant hand, ensuring that the animal’s leg remains extended.
Locate the medial (inner) or lateral (outer) saphenous vein and remove the hair using the electric shaver. Swab the intended puncture area with a cotton tipped applicator moistened with 70% ethanol and apply a thin film of petroleum jelly.
Using a sterile 27 G needle, puncture the blood vessel perpendicular to the skin at the most proximal visible area of the vein. Keep the needle in the vein for 2–3 s to allow the skin to stretch around the puncture site.
Collect blood (approximately 50–70 µL) in a heparinized capillary tube by gently touching the end of the capillary tube to the blood drop. Transfer the blood from the capillary to the blood collection tubes. Store tubes at room temperature until further processing.
Press the puncture site with a sterile gauze for at least 30 s or until bleeding stops. Transfer mice to cage and monitor for 10 min to ensure complete cessation of bleeding.
Processing and flow staining of peripheral blood
Transfer 50 µL of blood to a 96-well V-bottom plate and add 150 µL of PBS.
Centrifuge the plate at 1,065 × g for 3 min and pipette off supernatant. Meanwhile, prepare 1× RBC lysis buffer in distilled water and warm up in a 37 °C water bath.
First RBC lysis: Resuspend blood cell pellet in 180 µL of 1× RBC lysis buffer, mix well, incubate 3 min at room temperature, centrifuge at 1,065 × g for 3 min, and pipette off supernatant.
Second RBC lysis: Resuspend the cell pellet in 180 µL of 1× RBC lysis buffer, incubate 3 min at room temperature, centrifuge at 1,065 × g for 3 min, and pipette off supernatant.
Note: Cell pellet remains loosely attached during RBC lysis. To avoid cell loss, pipette off supernatant carefully.
First wash: Resuspend the cell pellet in 180 µL of PBS, centrifuge at 1,065 × g for 3 min, and pipette off the supernatant.
Second wash: Resuspend cell pellet again in 180 µL of PBS, centrifuge at 1,065 × g for 3 min, and pipette off the supernatant.
Dilute mouse Fc Block in PBS (see Table 2), add 25 µL/well, and incubate for 10 min at room temperature.
Table 2. Panel for weekly and endpoint engraftment testing
Type Marker Clone Fluor Dilution (2×) Supplier Cat. No.
SurfaceMouse Fc Block 2.4G2 - 1: 50 BD Biosciences 553142 FVD - eF780 1: 500 eBioscience 65-0865-18 mCD45 30-F11 AF700 1: 50 BD Biosciences 560510 hCD45 HI30 V500 1: 50 BD Biosciences 560777 hCD4 SK3 BV785 1: 50 BD Biosciences 563877 hCD8 RPA-T8 BV711 1: 100 BD Biosciences 563677 hCD3 UCHT1 BB515 1: 100 BD Biosciences 564466 hHLA-A2 BB7.2 APC 1: 100 Invitrogen 17-9876-42
IntracellularhFoxp3 236A/E7 PE-Cy7 1: 50 eBioscience 25-4777-42 hHelios 22F6 AF488 1: 50 BioLegend 137223 Meanwhile, prepare the surface staining mixture in PBS as per Table 2. Top up wells with 25 µL/well of staining mix, mix well, and incubate for 20 min at room temperature in the dark.
Top up each well with 150 µL of PBS, centrifuge at 1,065 × g for 3 min, and pipette off the supernatant.
Note: Optional additional steps for intracellular staining, e.g. to measure FOXP3 expression:
9a. Prepare 1× fix/perm buffer (one part fixation/permeabilization concentrate, three parts fixation/permeabilization diluent).
9b. Resuspend cell pellet in 100 µL of fix/perm buffer and incubate at room temperature for 45 min in the dark.
9c. Meanwhile, prepare 1× permeabilization buffer in distilled water.
9d. Top up the well containing cell pellet with 100 µL of permeabilization buffer, centrifuge at 1,065 × g for 3 min, and pipette off the supernatant.
9e. Resuspend cell pellet in 100 µL of permeabilization buffer a second time, centrifuge at 1,065 × g for 3 min, and pipette off the supernatant.
9f. Prepare intracellular staining mix in 1× permeabilization buffer as per the dilution in Table 2.
9g. Resuspend in 25 µL of staining mix and incubate for 40 min at room temperature in the dark.
9h. Top up wells with 180 µL of permeabilization buffer, centrifuge at 1,065 × g for 3 min, and pipette off the supernatant.
Top up each well with 180 µL of PBS, centrifuge at 1,065 × g for 3 min, and pipette off the supernatant.
Resuspend cells in 150 µL of FACS buffer (PBS + 1% FBS + 5 mM EDTA) and transfer cells to micro test tubes.
Note: To obtain absolute cell concentrations, 10,000 counting beads may be added to each micro test tube at this point. Make sure to vortex beads very well as they settle quickly. Set the voltages of forward scatter (FSC) and side scatter (SSC) such that both cells and beads are visible. Beads fluoresce strongly in both FITC/PE channels. Ensure bead fluorescence in these channels are on scale before acquiring samples.
Endpoint preparation of leukocytes for flow cytometry
Collection of cardiac blood and spleen
Prepare 1.5 mL Eppendorf tubes with 3 µL of heparin for cardiac blood and 1 mL of PBS for spleen.
Put mouse into surgical plane of anesthesia using isoflurane vaporizer gas anesthesia chamber.
Move mouse to nose cone, rinse a 1 mL syringe fitted with 27 G needle with heparin, perform cardiac puncture, and extract approximately 100 µL of blood.
Perform cervical dislocation and harvest spleen avoiding the pancreatic tissue (pancreatic enzymes may cause damage to T cells).
Processing and flow staining of cardiac blood and spleen
Blood:
Perform lysis and flow staining of 70 µL of blood as described in the section “Processing and flow staining of peripheral blood.”
Spleen:
Record the weight of the whole spleen, cut a small piece (approximately 15 mg) of spleen tissue, and mash on a pre-wet (with PBS) 70 µm cell strainer fitted on a 50 mL Falcon tube with a 1 mL syringe plunger. Rinse the filter with PBS until no visible particulate is present.
Note: Alternatively, mash the whole spleen, then take a portion of the cells (i.e. mash, centrifuge cells at 450 × g for 5 min, resuspend the cells in 1 mL PBS and take 20 µL for 1/50 of the total splenocytes).
Centrifuge the cell suspension at 450 × g for 5 min, discard supernatant by pouring, and transfer (approximately 200 µL) to a 96-well V-bottom plate.
Centrifuge the plate at 1,065 × g for 3 min and pipette off the supernatant.
Perform RBC lysis once by resuspending cell pellet in 180 µL of 1× RBC lysis buffer and incubate for 3 min at room temperature.
Resuspend cell pellet in 180 µL of PBS, centrifuge at 1,065 × g for 3 min, and pipette off supernatant.
Resuspend cell pellet again in 180 µL of PBS, centrifuge at 1,065 × g for 3 min, and pipette off and discard the supernatant.
Dilute mouse Fc block in PBS (see Table 2), add 25 µL/well, and incubate for 10 min at room temperature.
Meanwhile, prepare the staining mixture in PBS as per Table 2, add 25 µL of staining mix per well, and incubate for 20 min at room temperature in the dark.
Top up each well with 150 µL of PBS, centrifuge at 1,065 × g for 3 min, and pipette off the supernatant.
Wash cells by resuspending in 150 µL of FACS buffer, centrifuge at 1,065 × g for 3 min, and pipette off the supernatant.
Resuspend the cell pellet in 150 µL of FACS buffer and transfer to micro test tubes.
OPTIONAL: Processing and flow staining of laminar propria lymphocytes (LPL):
A major symptom of xenoGvHD is the manifestation of intestinal inflammation and colitis-like pathology. This can be further investigated by analyzing lymphocytes in the colon using the following protocol:
Collect colon: Open abdomen and collect entire colon from the rectum to the cecum. Gently remove fecal material by pushing along the colon towards the rectum. Place the colon into a 50 mL Falcon tube with 15 mL of cold RPMI supplemented with 10% FBS, 2 μM Gibco GlutaMax, 1× P/S, 25 mM HEPES, and 55 μM β-mercaptoethanol and store on ice.
Intestinal epithelial cell wash: Carefully discard the RPMI medium, add approximately 25 mL of PBS, and vortex for 2–3 s to wash the colon. Repeat this wash with an additional ~25 mL of PBS. Discard the PBS and add 15–20 mL of warm (37 °C) RPMI supplemented with 5% FBS, 1 mM DTT, and 1 mM EDTA. Vortex for 2–3 s and shake tubes at 37 °C for 10 min.
Intraepithelial lymphocyte wash: Carefully discard the RPMI/DTT mixture and add 15–20 mL of cold (4 °C) RPMI + 2% FBS. Vortex for 2–3 s to wash the colon, discard supernatant, and repeat this wash step with an additional 15–20 mL of RPMI + 2% FBS. Discard the supernatant and add 15–20 mL of warm (37 °C) RPMI supplemented with 5% FBS, 1 mM DTT, and 1 mM EDTA. Vortex for 2–3 s and shake tubes at 37 °C for 20 min.
Colon digestion: Carefully discard the RPMI/DTT mixture and add 15–20 mL of cold (4 °C) RPMI + 2% FBS. Vortex for 2–3 s to wash colon, discard supernatant, and repeat this wash step with an additional 15–20 mL of RPMI + 2% FBS. Discard supernatant and add 14 mL of warm (37 °C) RPMI supplemented with 0.5 mg/mL collagenase VIII + 20 µg/mL DNase I. Shake at 37 °C for 30 min to digest the colon.
Pour the media and digested colon over a fresh 70 µm cell strainer into a fresh 50 mL Falcon tube. Mechanically disrupt the remaining colon with a syringe plunger against the cell strainer and wash the cell strainer with additional cold RPMI + 2% FBS to a final volume of 35 mL. Pellet the cells at 500 × g for 10 min.
Lymphocyte enrichment: Make an isotonic Percoll solution (nine parts Percoll to one part 10× PBS) and use this to dilute with 1× PBS to yield 40% and 80% Percoll solutions. Add 3 mL of 80% Percoll to 15 mL Falcon tubes. Resuspend the cell pellet from step 5 in 4 mL of 40% Percoll and carefully overlay onto the 80% Percoll. Centrifuge at 652 × g at room temperature with brake off.
Remove the lipid-like top layer to prevent contamination and collect the interphase containing LPLs (approximately 3 mL) into a fresh 15 mL tube.Top up with PBS to wash and pellet the cells at 652 × g for 5 min at 4 °C. Repeat this wash step and proceed to stain the enriched LPLs.
Dilute mouse Fc block in PBS (see Table 2), add 25 µL/well, and incubate for 10 min at room temperature.
Meanwhile, prepare the staining mixture in PBS as per Table 2, top up with 25 µL/well of staining mix, and incubate for 20 min at room temperature in the dark.
Top up each well with 150 µL of PBS, centrifuge at 1,065 × g for 3 min, and pipette off the supernatant.
Wash cells by resuspending in 150 µL of FACS buffer, centrifuge at 1,065 × g for 3 min, and pipette off the supernatant.
Resuspend the cell pellet in 150 µL of FACS buffer and transfer to micro test tubes. Add 10,000 counting beads if an absolute cell count is desired.
Data analysis
Flow cytometry data analysis
Data presented here were acquired using a BD LSRFortessaTM X-20 flow cytometer and analyzed in FlowJo software. However, different flow cytometers with similar configurations can also be used. Data should be analyzed as shown in Figure 2. From total live events, human cell engraftment can be calculated as a proportion of total (human and mouse) CD45+ cells. With injection of PBMC alone, human cell engraftment is expected to range from 20% to 30% by day 21 post injection.
Note: Before using PBMC for an actual experiment it is strongly recommended to pre-test their xenoGvHD-inducing capacity in a pilot study.
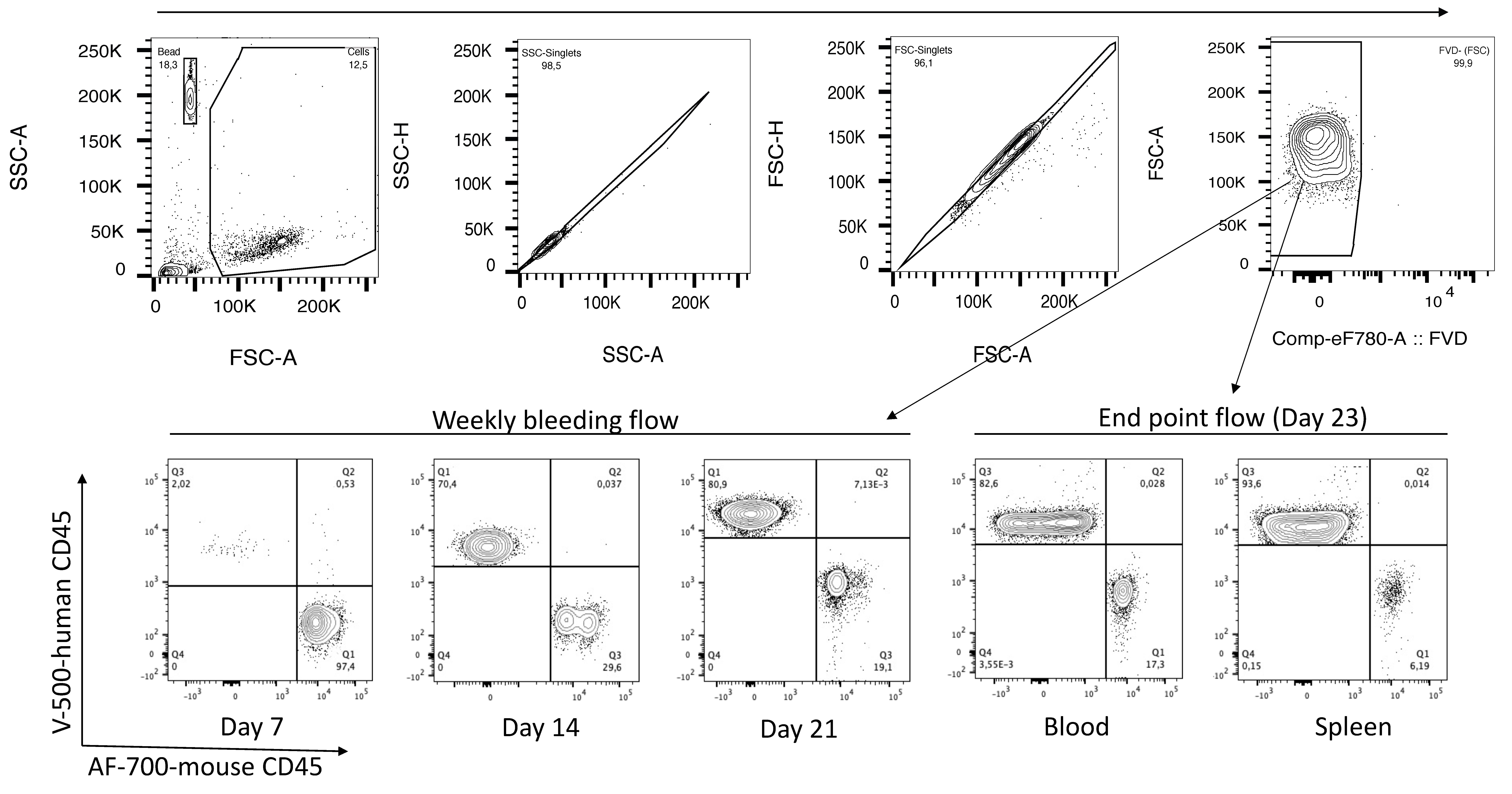
Figure 2. Gating strategy for calculating PBMC engraftment. The voltage should be set such that the forward and side scatter (FSC-A vs. SSC-A) plot shows both total cells and beads (first plot). From total cells, gate singlets and live cells (FVD-negative fraction). From total live cells, gate mouse CD45 (AF-700-mCD45) and human CD45 (V500-hCD45) and calculate the % human CD45 cells in the upper left quadrant. The first flow-plot (SSC-A vs. FSC-A) is showing.
Absolute cell count
Vortex beads for 30 s and add 10,000 beads for blood and 20,000 beads for spleen samples before running sample in flow cytometer. We adjusted the bead concentration such that 10 µL contains 10,000 beads.
Adjust the SSC and FSC voltage to detect both beads and cells (see Figure 2, top left). Draw a gate on the counting beads, display on a flow plot with FITC vs. PE, and gate on the double positive population (Figure 3).
Use the count statistics in the data analysis software (FlowJo) to determine the cell concentration using the equation below.
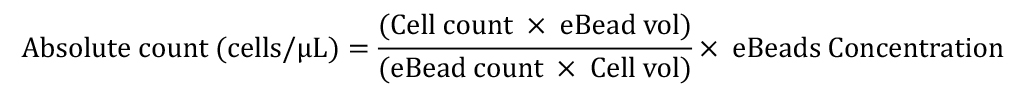
Note: Collect at least 7,000–8,000 bead events to obtain statistically significant results.
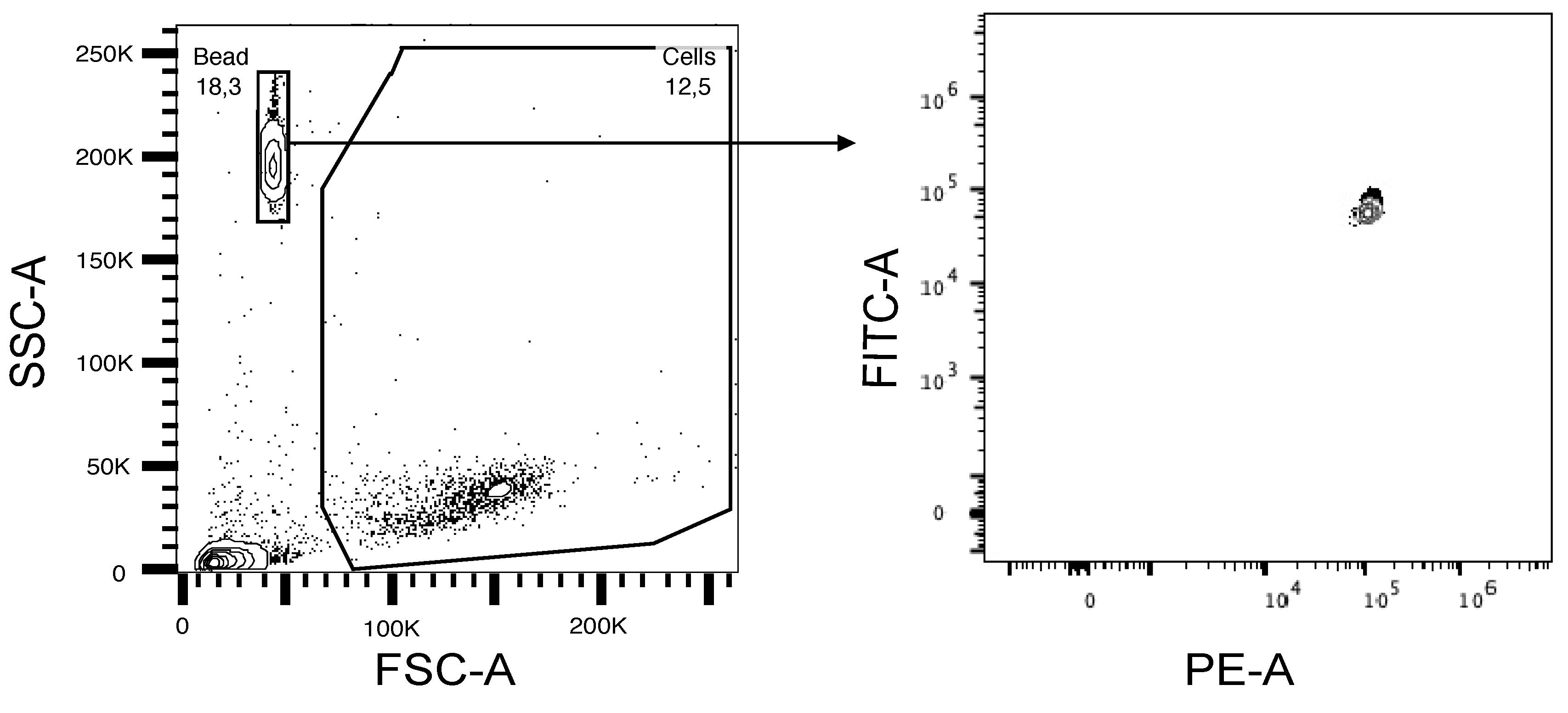
Figure 3. Flow plot showing the gating of cells and counting beads in SSC vs. FSC and FITC+PE+ beads. Absolute cell number calculation is based upon the PE/FITC double positive event count.
Thymic Tregs delay the development of xenoGvHD
As an example of how this model can be used to test new therapies, in Figure 4 we show an example of Treg-mediated suppression of xenoGvHD.
Preparation of thymic Tregs: Thymic Tregs were prepared according to MacDonald et al. (2019). Briefly, thymocytes were isolated by mechanical dissociation of thymic tissue followed by CD25-positive and CD8-negative selection. The protocol yields >90% pure CD4+CD25+Foxp3+ thymic Tregs.
Preparation of PBMCs: Frozen PBMC were thawed following the process described in section B above.
Cell injection: Resuspend both PBMCs and Tregs at a cell concentration of 70 × 106/mL in PBS and keep them separate. At the time of injection, mix 100 µL of PBMC and 100 µL of Tregs and inject 200 µL of the mixed cell suspension via the tail vein following the method described in the PBMC injection section above.
Note: If Tregs are allogeneic to PBMCs, it is useful to have a known HLA-mismatch to facilitate cell tracking. We routinely phenotype PBMCs and Tregs for HLA-A2, -A3, and -A24 to facilitate this process. Allogeneic Tregs diminish the engraftment of human PBMCs whereas autologous Tregs show minimal impact on PBMC engraftment (Dawson et al., 2019). Tregs engineered with different types of receptors (e.g., chimeric antigen receptor) may have greater effects on PBMC engraftment.
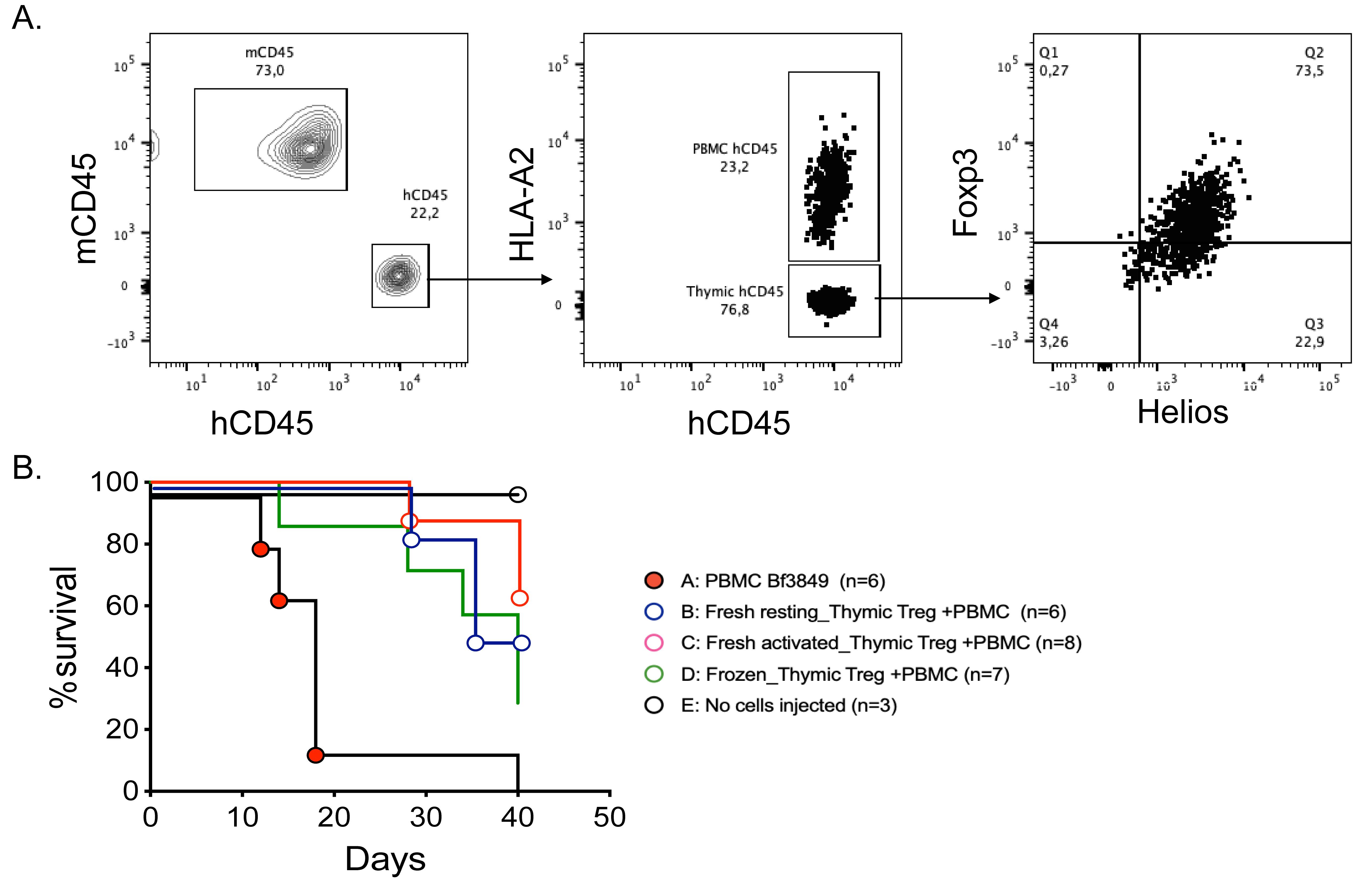
Figure 4. Suppression of xenoGvHD by thymic Tregs. Seven million HLA-A2+ PBMCs and seven million HLA-A2- thymic Tregs were injected into irradiated NSG mice. The infused Tregs were either freshly isolated and rested, freshly isolated and activated, or previously frozen. (A). Flow plot showing the gating strategy for detecting HLA-A2+ PBMCs and HLA-A2- thymic Tregs on day 7 post cell injection. (B). Kaplan–Meier survival curve showing that infusion of Tregs delayed the onset of xenoGvHD.
OPTIONAL: Histology of organs
To detect cellular infiltration, perform histology of various organs (lungs, liver, heart, intestine, kidney, skin, etc.). The onset of a higher degree of xenoGvHD shows massive infiltration of human-CD45 positive cells. For details, see Dawson et al. (2019).
Acknowledgments
This work was supported by funding from the Canadian Institutes of Health Research.
Competing interests
MKL has received research funding from Sangamo Therapeutics, Bristol-Myers Squibb, Pfizer, Takeda, and CRISPR Therapeutics for work unrelated to this study. All other authors declare no competing interests.
Ethics
Researchers must obtain appropriate training and ethical approval from the institutional animal ethical committee before conducting any mouse experiment. Data presented here were obtained from experiments conducted with the approval of the University of British Columbia Animal Care Committee (Protocol: A18-0180).
References
- Adigbli, G., Menoret, S., Cross, A. R., Hester, J., Issa, F. and Anegon, I. (2020). Humanization of Immunodeficient Animals for the Modeling of Transplantation, Graft Versus Host Disease, and Regenerative Medicine. Transplantation 104(11): 2290-2306.
- Dawson, N. A., Lamarche, C., Hoeppli, R. E., Bergqvist, P., Fung, V. C., McIver, E., Huang, Q., Gillies, J., Speck, M., Orban, P. C., et al. (2019). Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells. JCI Insight 4(6). e123672.
- Ehx, G., Somja, J., Warnatz, H. J., Ritacco, C., Hannon, M., Delens, L., Fransolet, G., Delvenne, P., Muller, J., Beguin, Y., Lehrach, H., Belle, L., Humblet-Baron, S., Baron, F. (2018). Xenogeneic Graft-Versus-Host Disease in Humanized NSG and NSG-HLA-A2/HHD Mice. Front Immunol 9:1943.
- Ferrara, J. L., Levine, J. E., Reddy, P. and Holler, E. (2009). Graft-versus-host disease. Lancet 373(9674): 1550-1561.
- Gibson, B. W., Boles, N. C., Souroullas, G. P., Herron, A. J., Fraley, J. K., Schwiebert, R. S., Sharp, J. J. and Goodell, M. A. (2015). Comparison of Cesium-137 and X-ray Irradiators by Using Bone Marrow Transplant Reconstitution in C57BL/6J Mice. Comp Med 65(3):165-72.
- Gschweng, E., De Oliveira, S. and Kohn, D. B. (2014). Hematopoietic stem cells for cancer immunotherapy. Immunol Rev 257(1): 237-249.
- Khosravi-Maharlooei, M., Madley, R., Borsotti, C., Ferreira, L. M. R., Sharp, R. C., Brehm, M. A., Greiner, D. L., Parent, A. V., Anderson, M. S., Sykes, M., et al. (2022). Modeling human T1D-associated autoimmune processes. Mol Metab 56: 101417.
- King, M. A., Covassin, L., Brehm, M. A., Racki, W., Pearson, T., Leif, J., Laning, J., Fodor, W., Foreman, O., Burzenski, L., et al. (2009). Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol 157(1): 104-118.
- Loiseau, P., Busson, M., Balere, M. L., Dormoy, A., Bignon, J. D., Gagne, K., Gebuhrer, L., Dubois, V., Jollet, I., Bois, M., et al. (2007). HLA Association with hematopoietic stem cell transplantation outcome: the number of mismatches at HLA-A, -B, -C, -DRB1, or -DQB1 is strongly associated with overall survival. Biol Blood Marrow Transplant 13(8): 965-974.
- MacDonald, K. N., Piret, J. M. and Levings, M. K. (2019). Methods to manufacture regulatory T cells for cell therapy. Clin Exp Immunol 197(1): 52-63.
- Walsh, N. C., Kenney, L. L., Jangalwe, S., Aryee, K. E., Greiner, D. L., Brehm, M. A., Shultz, L. D. (2017). Humanized Mouse Models of Clinical Disease. Annu Rev Pathol 12:187-215.
- Yano, J., Lilly, E. A., Noverr, M. C. and Fidel, P. L. (2020). A Contemporary Warming/Restraining Device for Efficient Tail Vein Injections in a Murine Fungal Sepsis Model. J Vis Exp(165): e61961.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Haque, M., Boardman, D. A., Lam, A. J., MacDonald, K. N., Sanderink, L., Huang, Q., Fung, V. C., Ivison, S., Mojibian, M. and Levings, M. K. (2022). Modelling Graft-Versus-Host Disease in Mice Using Human Peripheral Blood Mononuclear Cells. Bio-protocol 12(23): e4566. DOI: 10.21769/BioProtoc.4566.
Category
Medicine > Inflammation
Immunology > Host defense
Cell Biology > Cell Transplantation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link