- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Evaluation of Mitochondrial Turnover Using Fluorescence Microscopy in Drosophila
Published: Vol 12, Iss 17, Sep 5, 2022 DOI: 10.21769/BioProtoc.4498 Views: 2798
Reviewed by: Chiara AmbrogioMaría Victoria MartinMichael Enos

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Preparation of Drosophila Polytene Chromosomes, Followed by Immunofluorescence Analysis of Chromatin Structure by Multi-fluorescence Correlations
Terra M. Kuhn [...] Maya Capelson
Jul 5, 2020 9913 Views
Labeling and Tracking Mitochondria with Photoactivation in Drosophila Embryos
Sayali Chowdhary and Richa Rikhy
Mar 5, 2022 4321 Views
Isolation of Intact Mitochondria From Drosophila melanogaster and Assessment of Mitochondrial Respiratory Capacity Using Seahorse Analyzer
Christopher M. Groen and Anthony J. Windebank
Feb 5, 2025 2241 Views
Abstract
Mitochondrial dysfunction is associated with perturbations in the cellular oxidative status, changes in energy production and metabolic rate, and the onset of pathological processes. Classic methods of assessing mitochondrial dysfunction rely on indirect measures, such as evaluating mitochondrial DNA copy numbers, or direct but more costly and skilled techniques, such as electron microscopy. The protocol presented here was recently implemented to evaluate mitochondrial dysfunction in response to insecticide exposure in Drosophila melanogaster larvae, and it relies on the use of a previously established MitoTimer mutant strain. MitoTimer is a genetically engineered mitochondrial protein that shows green fluorescence when newly synthetized, irreversibly turning into red as mitochondria age. The protocol described here allows for the easy and direct assessment of shifts in mitochondrial turnover, with tissue-specific accuracy. This protocol can be adapted to assess changes in mitochondrial turnover in response to drugs, rearing conditions, and/or mutations in larva, pupa, or adult fruit flies.
Keywords: Mitochondrial turnoverBackground
Mitochondria are dynamic organelles that generate most of the chemical energy (ATP) required to sustain cellular activity in eukaryotes (Chan, 2020). These organelles are also involved in the activation of cell death by apoptosis, regulation of Ca2+ homeostasis, synthesis of phospholipids and β-oxidation of fatty acids, as well as the activation, differentiation, and survival of immune cells (Nunnari and Suomalainen, 2012; Chan, 2020). Given the central role of mitochondria in a diverse range of vital cellular activities, the balance between mitogenesis (production of new mitochondria by growth and division) and mitophagy (selective degradation of old/defective mitochondria by autophagy) is tightly regulated by the cell. Whilst mitogenesis is coordinated with vascularization and enhanced oxygen delivery for optimal energy production (Nunnari and Suomalainen, 2012), mitophagy ensures proper mitochondrial function by preventing that defective organelles accumulate and perpetuate damage to healthy ones (Bell and Tower, 2021). This turnover maintains cellular homeostasis, and it may lead to degenerative and age-related disorders when impaired (Chan, 2020; Bell and Tower, 2021).
As we age, mitochondrial gene expression, mitochondrial metabolic rates, and mitochondrial turnover decrease (Bell and Tower, 2021). Such phenomenon is conserved in different species (Landis et al., 2004; Frenk and Houseley, 2018; Leboutet et al., 2020). Mitochondrial defects arise mostly from damage caused by reactive oxygen species (ROS). Many ROS are by-products of oxidative phosphorylation, as leaked electrons from the electron transport chain react with oxygen creating superoxide, the primary mitochondrial ROS (Shadel and Horvath, 2015). Distinct ROS molecules perform a plethora of cellular signalling functions under natural conditions, regulating physiology and metabolism (Valko et al., 2007; Shadel and Horvath, 2015). ROS only become a problem under situations of oxidative stress, which is caused by an exacerbated ROS generation, or reduced cellular antioxidant capacity. In this scenario, ROS can damage lipids, proteins, and nucleic acids, affecting cell homeostasis. Given that great part of ROS are produced in mitochondria, these organelles are prone to oxidative damage, triggering a cycle where damaged mitochondria lead to more ROS generation (Islam, 2017; Pizzino et al., 2017). Mitochondrial dysfunction and mitochondrial ROS are considered key players in aging and aging-associated neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases, or multiple sclerosis (Liu et al., 2015; Islam, 2017; Bell and Tower, 2021; Johnson et al., 2021).
A number of methods exist to measure mitochondrial (dys)function. A common indirect measure for mitochondria abundance is to detect mitochondrial DNA copy number (Filograna et al., 2021), but that provides no accurate measure of mitochondrial stress. Assays that measure ATP levels (Brand and Nicholls, 2011) or the activity level of mitochondrial aconitase (Yan et al., 1997) are also methods of estimating changes in mitochondrial metabolic rate or oxidative status. However, such methods, or the ones used to assess mitochondrial respiration (Smolina et al., 2017), require the use of isolated cells or isolated mitochondria. That may cause damage to the organelles during the isolation process, or may remove them from the cellular context (Brand and Nicholls, 2011). Other approaches include more skilled and costly techniques, such as electron microscopy for assessing mitochondria number and morphology (Duranova et al., 2020). A range of fluorescent probes that target the generation of ROS and other metabolites in mitochondria, and changes in mitochondrial membrane potential, are also available (Chu et al., 2021). However, these protocols are more laborious, requiring extra steps to incorporate the probes. In comparison to these methods, the use of genetically engineered MitoTimer mutants offers an easy readout of the levels of newer and older/stressed mitochondria that requires no organelle isolation, no additional staining, or the use of probes, and is less costly. MitoTimer is based on a Timer mutant protein of DsRed engineered with a mitochondrial-targeting sequence. This protein shows green fluorescence when newly synthetized, irreversibly turning into red over time (Ferree et al., 2013; Hernandez et al., 2013; Laker et al., 2014). While the green signal is mostly determined by MitoTimer synthesis, mitochondrial biogenesis, or protein import, the red signal is mainly affected by stress, degradative pathways, mitochondrial proteases, or the ubiquitin–proteasome system (Ferree et al., 2013).
Two recent studies on the mechanisms of action of low insecticide doses used MitoTimer reporter flies to assess the effects of insecticide exposure on mitochondrial turnover (Martelli et al., 2020; Martelli et al., 2022). These studies revealed that the insecticides, spinosad and imidacloprid, caused a rise of both newer and older/stressed mitochondria, suggesting an increase in both mitogenesis and mitochondrial stress. This hypothesis was further corroborated by the observation by electron microscopy of a higher overall number of mitochondria, as well as defective mitochondria, and increased levels of ROS by Dihydroethidium (DHE) staining in insecticide exposed flies (Martelli et al., 2020; Martelli et al., 2022). The protocol used in these studies is described here. This protocol can be promptly adapted for testing the effect of drugs, rearing conditions, or mutations on mitochondrial stress at different life stages of Drosophila. MitoTimer reporters are also available in C. elegans, mice, and mouse cell culture (Laker et al., 2014; Gottlieb and Stotland, 2015).
Materials and Reagents
1.7 mL microtubes (Axygen, catalog number: MCT-175-C)
48-well Nunc cell plate (Thermo Scientific, catalog number: 150687)
Micropipette 200 µL tips (Axygen, catalog number: T-200-Y)
Double-sided tape
Cotton (Genesee Scientific, catalog number: 51-101)
25 × 95 mm polystyrene Drosophila culture vials (Genesee Scientific, catalog number: 32-109RL)
Microscopy slides (Westlab, catalog number: 663-249)
Cover slips (Trajan, catalog number: 471112440M)
90 mm Petri dishes
MitoTimer reporter Drosophila strain (Bloomington Drosophila Stock Center, # line: 57323)
Neuronal Gal4 driver Drosophila strain (Bloomington Drosophila Stock Center, # line: 458)
Phosphate-buffered saline (PBS) (Sigma-Aldrich, catalog number: P5493)
16% Formaldehyde solution (Thermo Fisher Scientific, catalog number: 28908)
Spinosad (Sigma-Aldrich, catalog number: 33706)
Dimethyl Sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 276855-100ML)
Vectashield 10 mL Mounting medium (Vector, catalog number: VEH1000)
Analytical standard sucrose (Merk, product number: 47289)
Fly media (1 L) (see Recipes)
Apple juice-agar plates (1 L) (see Recipes)
Tegosept (250 mL) (see Recipes)
Acid mix (250 mL) (see Recipes)
20% Sucrose solution (200 mL) (see Recipes)
5% Sucrose solution (200 mL) (see Recipes)
1,000 ppm spinosad solution in DMSO (see Recipes)
4% Formaldehyde solution (see Recipes)
Equipment
Dissecting forceps (Fine Science Tools, Dumont #5 Forceps, catalog number:1195-10)
Brush (Artist First Choice Taklon round size 3)
Stereo microscope (Zeiss, model: Stemi 2000-C)
Confocal microscope (Leica, model: SP8 Confocal microscope)
Delicate task wipers (Kimtech Science Kimwipes, manufacturer code: 34120A)
Micropipette P200 (Gibson, catalog number: FA10005M)
Incubator 25 °C (Thermoline Scientific)
500 mL laboratory bottles (Sigma-Aldrich, catalog number: Z305197-10EA)
Agitator (Benchmark Scientific Inc, Super Nutation Mixer, catalog number: B3D 1320)
Stove
Software
ImageJ/FIJI (NIH, https://fiji.sc/)
Excel (Microsoft)
R (The R Foundation, https://www.r-project.org/)
Inkscape (https://inkscape.org/)
Procedure
Drosophila rearing
Set a vial containing fly media with twenty Neuronal Gal4 driver Drosophila adult virgin females and five MitoTimer reporter Drosophila adult males.
Keep vials in an incubator at 25 °C, and transfer adult flies to a new vial containing fly media every 24 h.
Set 2 to 4 vials with adults laying eggs per day, to collect enough larvae of the desired stage (early third instar larvae are used here).
Larvae collection
To collect early third instar larvae, wait 4 to 5 days from egg laying, depending on larvae population density per vial.
Recover larvae from the food using the sucrose extraction method (Nichols et al., 2012). Select a vial containing larvae, and cover half of it with 20% sucrose solution (Figure 1A).
Using a brush, gently disrupt the top food layer and wait for a couple of minutes. The larvae will float, and the food will sink back to the bottom.
Using a brush, collect the larvae and transfer them to an apple juice-agar plate (Figure 1B).
Insecticide exposure
Using the micropipette, load a 48-well Nunc plate with 200 µL of 5% sucrose solution per well.
Under the stereo microscope, select early third instar larvae (Figure 1C) and, using a pair of forceps, gently transfer 25 larvae per well (Figure 1D).
For the insecticide exposure, create a 5x insecticide solution by diluting a 1,000 ppm insecticide stock solution in 5% sucrose solution (or equivalent dose of DMSO for controls) (Denecke et al., 2015).
Add 50 µL of the 5× stock solution to each well containing larvae to be exposed, and give the plate a gentle swirl (Figure 1D).
Keep the plate with larvae under insecticide exposure at 25 °C and protected from light for 2 h (or desirable number of hours), until exposure is over.
Once exposure time is over, using a pair of forceps, gently transfer larvae to a new well containing 250 µL of 1× PBS (Figure 1E).
Tissue dissection and fixation
Transfer larva from the PBS containing well to a microscopy slide containing a drop of 1× PBS.
Under the stereo microscope, begin the dissection. Using two pairs of forceps, hold the larval body at the midpoint, and pull the anterior and posterior regions apart (Figure 1F).
Using a pair of forceps, gently transfer the anterior body section to a microtube containing 500 µL of 4% formaldehyde solution (Figure 1G).
Keep the microtubes at slow agitation in a bench agitator for 20 min, to fix the sample.
With a micropipette, slowly and completely remove the fixative solution, whilst avoiding touching the sample. Dispose of the toxic formaldehyde waste appropriately.
Gently add 500 µL of 1× PBS back to the microtube, to avoid damaging the sample, and place the microtube back on the agitator for another 5 min.
Repeat the previous step two more times.
Under the stereo microscope, add a drop of 1× PBS to a new microscopy slide. Using a pair of forceps, gently transfer the fixed sample from the microtube onto the slide (Figure 1H).
Conclude the dissection by holding the anterior body section sideways with two pairs of forceps, and gently pull them apart from each other to tear the cuticle. Once the brain is located, use the forceps to clear the surrounding tissues. If PBS starts to dry out, add more of it accordingly.
Mounting slides for fluorescence microscopy
Clear the remaining tissue from the slide, leaving only the brain.
Using a delicate task wiper’s tip, carefully drain the excess of PBS. Moistening the wiper’s tip in distilled water before touching the PBS, as far away from the fixed sample as possible, will reduce its dragging capacity, and prevent the sample from being sucked into the wiper.
Add a small drop of Vectashield on the top of the fixed sample, only enough to cover it. Using a pair of forceps, if necessary, adjust sample orientation.
Cut two small pieces of double-sided tape, and place them flanking the sample in Vectashield.
Place the coverslip on the top, aligning its border with the double-sided tape (Figure 1I).
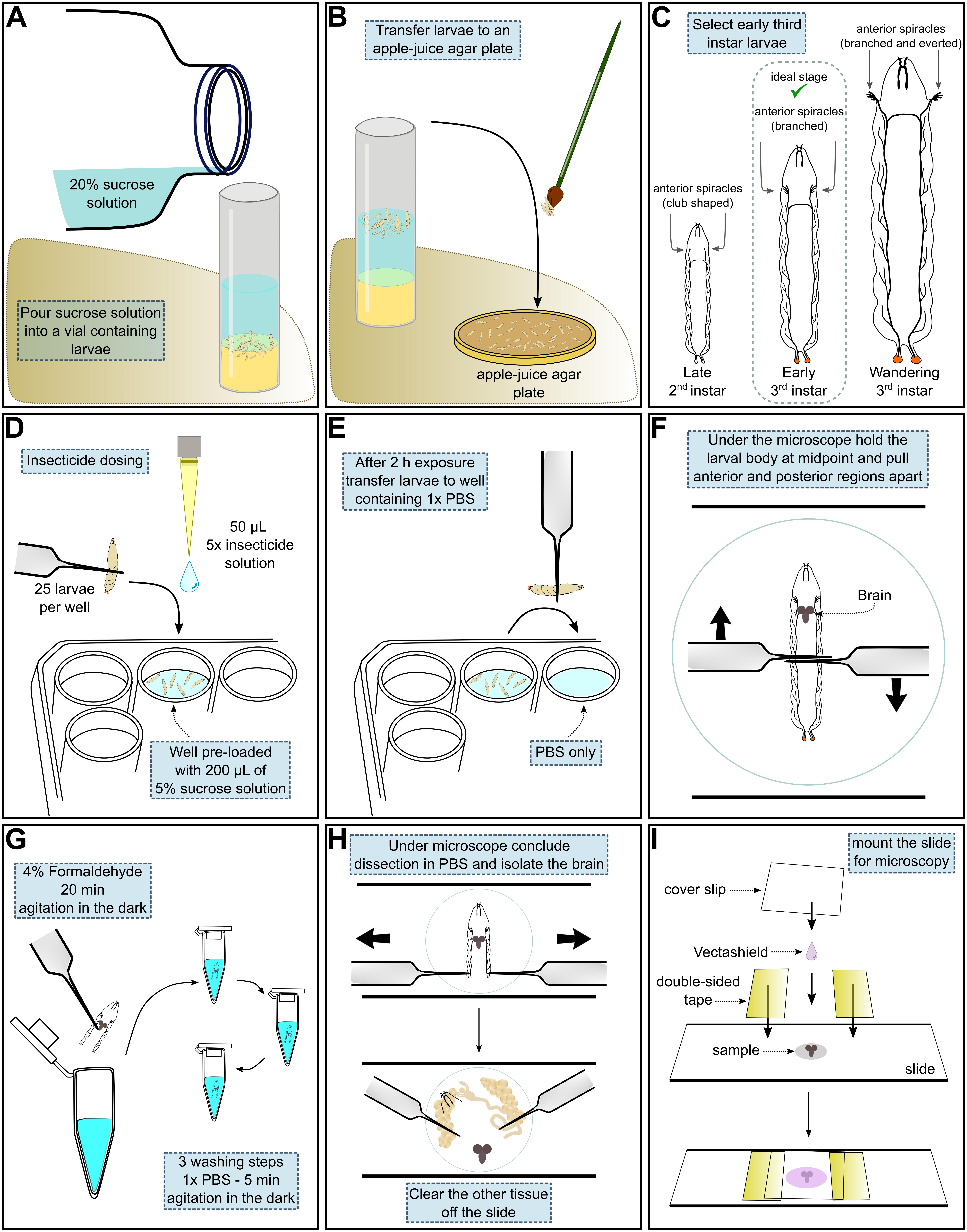
Figure 1. Experimental procedure. A. Use the sucrose extraction method to recover larvae from the vial. B. Using a brush, transfer larvae to an apple-juice agar plate. C. Selected early third instar larvae. D. Transfer 25 larvae to a well pre-loaded with 5% sucrose solution, and expose them to insecticide. E. Once exposure is over, transfer larvae to a new well containing 1× PBS solution. F. Under the microscope, begin dissection by separating anterior and posterior body parts. G. Transfer the anterior body part to a microtube containing fixative solution. After fixing the sample, perform three washing steps by replacing the solution in the microtube with 1× PBS. H. Under the microscope, conclude dissection, and isolate the brain from the other tissues. I. Mount the slide for microscopy using double-sided tape, Vectashield, and a cover slip.Imaging
Proceed to image acquisition with a fluorescent microscope immediately.
To ensure consistency and accuracy in the measurements, set optimal values of laser power and gain with a control sample, and maintain the same settings across all samples.
Once the desired area is localized, acquire both green (excitation/emission 488/518 nm) and red (excitation/emission 543/572 nm) signals at 200× magnification.
Acquire at least 20 images across the z-stack for every sample.
Data analysis
Analyzing microscopy images on ImageJ
Open the image files on ImageJ and use the freehand selection tool to create a selection area around the area of interest (e.g., whole brain, ventral nerve cord, or optic lobes—used here).
On the top menu, under Analyze, select Measure.
Copy the mean values of fluorescence intensity generated to an Excel spreadsheet.
For every sample, acquire three independent measurements along the z-stack that are representative of the sample signal.
Use the same selection area and same z-stack images for both green and red MitoTimer signal measurements per sample.
Obtain measurements from the same regions in the different samples.
Use at least 10 individual samples per treatment condition for a consistent evaluation of changes on mitochondrial turnover.
Identify microscopy images that are representative of control and treated conditions and, using a suitable program (e.g., Inkscape), organize these images for publication (Figure 2A).
Statistical analyses
On an Excel spreadsheet, organize the data in columns to show treatment condition, nature of the signal, sample replicate number, and signal values (Figure 2B).
Save the file as mitotimer.csv
Open the software R and download the packages lme4 and car
To perform statistical analysis, use the following script:
library(lme4)
library(car)
mitotimer=read.csv(file.choose("mitotimer"),header=TRUE)
model<-lmer(value~signal*treatment+(1|replicate),REML=FALSE,na.action= na.omit,data=mitotimer)
summary(model)
car::Anova(model)
The script generates a generalized linear mixed model that compares treatment and MitoTimer signal values. It also uses the three independent measurement replicates per sample as a random effect. The analysis is followed by an ANOVA test that provides test statistics and the p-values.
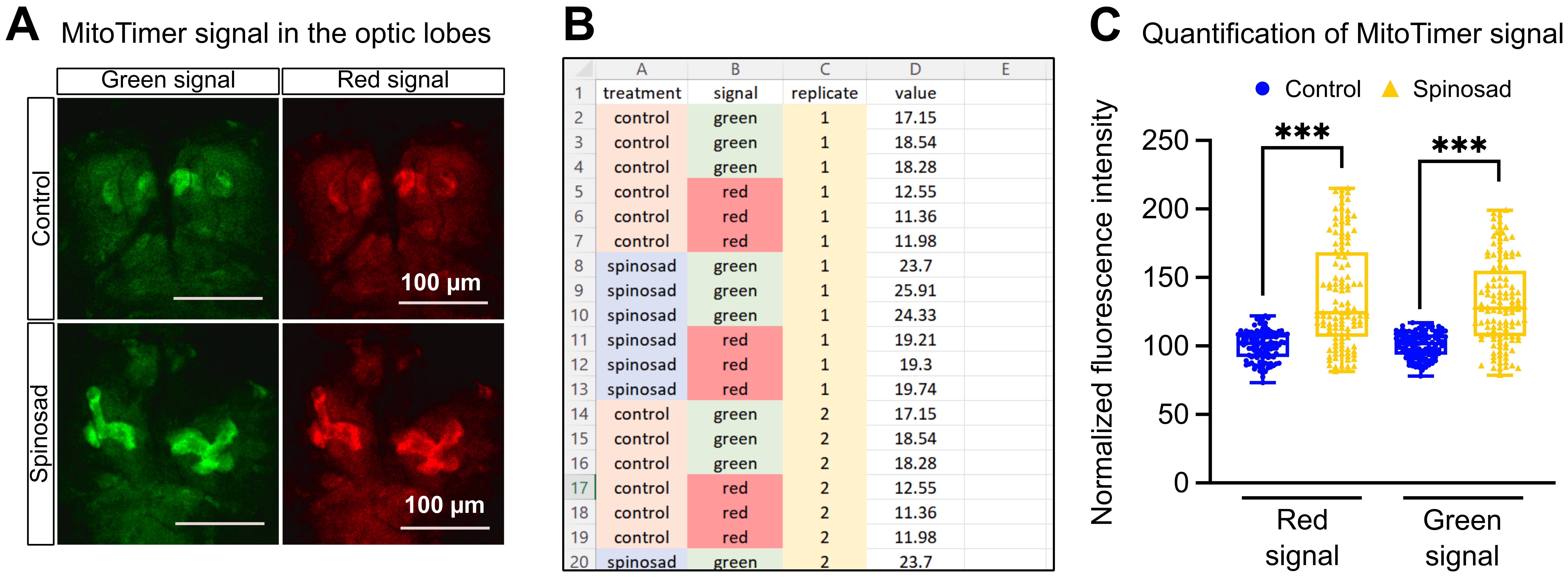
Figure 2. Data analysis and representation of mitochondrial turnover results. A. Example confocal microscopy image of green and red MitoTimer signal in the optic lobes of third instar larval brains. Control and spinosad exposed individuals are represented. B. Example of how to organize a .csv table containing the MitoTimer fluorescence signals measured using ImageJ. This table is used to perform the statistical analysis in R. C. Normalized mean fluorescence intensity of MitoTimer signals in optic lobes (n = 20 larvae/treatment; three image sections/larva). Generalized linear mixed model followed by ANOVA. (***) p-value <0.001.Data normalization and plotting
To facilitate data visualization and plotting, represent the data in terms of normalized mean fluorescence intensity.
Independently for green and red MitoTimer signals, calculate the mean fluoresce intensity of control samples. For a given control group, divide each individual sample value by the group mean and then multiple it by 100. Using that same group mean value, perform the same operation for the respective treated samples. At the end, control groups should have mean green and red signal values of 100, and the treated samples should have values normalized to their respective controls.
Using a suitable program (e.g., R) graphically represent the data in the form of bar plots or box plots, ideally including dot plots (Figure 2C).
Notes
Spinosad was used here to test its capacity to generate oxidative stress. However, this protocol can be applied to the same end for testing the effect of other drugs, rearing conditions, and/or mutations on mitochondrial turnover.
Given the exposure method used here, early third instar larvae are preferable, as late wandering third instars may crawl out of the wells or start pupation during exposure, and first or second instars would offer extra challenges for dissection, given their smaller size. However, other life stages may be selected depending on the research focus, including pupal and adult stages.
Image acquisition under the confocal microscope should be performed immediately after microscopy slides are ready, to guarantee sample integrity and the reliability of the signal acquired.
If performing insecticide/drug exposures for a fixed time with large sample groups, do not start exposure of all groups at the same time. Instead, start the exposure of each group at a different time, creating regular intervals of 10 or 15 min between them (or as required). That will allow time for sample dissection and microscopy of smaller sample batches, while ensuring that all individuals are exposed to roughly the same time.
Handle formaldehyde inside the fume hood, as it is toxic, and dispose of its waste as required.
More than one sample can be transferred to the same microtube with 4% formaldehyde solution. However, if doing so, transfer all these samples from the dissection slide into the microtube at once, to ensure that they are all fixed for the same amount of time.
Here, an elav-Gal4 driver was used to drive MitoTimer expression in neurons. Other Gal4 drivers can be used to different ends. Drosophila Gal4 driver strains can be found on the website of Bloomington Drosophila Stock Center (https://bdsc.indiana.edu/).
Recipes
Fly media (1 L)
Water 800 mL
Maize meal 60 g
Dried active yeast 15 g
Agar 6 g
Acid mix 7.5 mL
Tegosept 5 mL
Set the stove heat to high and in a pot add water and agar. Simmer and bring it to boil. After boiling the mixture for a couple of minutes, reduce the heat to medium, and add maize meal and dried active yeast. Simmer the mixture until homogenous, and turn off the heat. Wait a couple of minutes before adding acid mix and Tegosept while simmering. Dispense around 10 mL of fly media per vial, and wait for 12 h for it to set before closing the vials with cotton. Vials with fly media can be kept in a 4 °C fridge for a month but must be brought to room temperature before usage.
Apple juice-agar plates (1 L)
Water 720 mL
Agar 20 g
Apple juice 200 mL
Brewer’s yeast 7 g
Glucose 52 g
Sucrose 26 g
Tegosept 6 mL
Set the stove heat to high and, in a pot, add water and agar. Simmer and bring it to boil. After boiling the mixture for a couple of minutes, reduce the heat to medium and add apple juice, brewer’s yeast, glucose, and sucrose. Simmer the mixture until homogeneous, and turn off the heat. Wait a couple of minutes before adding Tegosept, and do it while simmering. Dispense enough media to fully cover the petri dish. Wait for it to solidify, and keep it upside down in the fridge at 4 °C; it must be brought to room temperature before usage.
Tegosept (250 mL)
Distilled water 137 mL
Propionic acid 103 mL
Orthophosphoric acid 10 mL
Add distilled water, propionic acid, and orthophosphoric acid into a 500 mL glass bottle. The solution can be kept at room temperature.
Acid mix (250 mL)
Ethanol 95% 250 mL
p-Hydroxybenzoic acid methyl ester 25 g
Add ethanol, distilled water, and p-Hydroxybenzoic acid methyl ester into a 500 mL glass bottle. The solution can be kept at room temperature.
20% Sucrose solution (200 mL)
Distilled water 160 mL
Analytical standard sucrose 40 g
Add distilled water and sucrose into a 500 mL glass bottle and give it a swirl until completely dissolved. It can be kept in the 4 °C fridge for a month, but must be brought to room temperature before usage.
5% Sucrose solution (200 mL)
Distilled water 190 mL
Analytical standard sucrose 10 g
Add distilled water and sucrose into a 500 mL glass bottle and give it a swirl until completely dissolved. It can be kept in the 4 °C fridge for a month but must be brought to room temperature before usage.
1,000 ppm spinosad solution in DMSO
DMSO 1 mL
Spinosad 1 mg
Add DMSO and Spinosad into a 1.7 mL microtube and vortex vigorously for a few minutes until solubilized. Store the insecticide dilution in a freezer (-20 °C) protected from light to prevent degradation. Create small volume aliquots (approximately 100 µL each) to avoid multiple freeze-thaw cycles.
4% Formaldehyde solution
Distilled water 375 µL
16% Formaldehyde solution 125 µL
Add distilled water and 16% formaldehyde to a 1.7 mL microtube.
Acknowledgments
The author was supported by a Victorian Latin America Doctoral Scholarship (Victorian Government and the University of Melbourne), an Alfred Nicholas Fellowship (University of Melbourne), and a University of Melbourne Faculty of Science Travelling Scholarship.
Original research paper: Martelli, F., Hernandes, N. H., Zuo, Z., Wang, J., Wong, C. O., Karagas, N. E., Roessner, U., Rupasinghe, T., Robin, C., Venkatachalam, K., et al. (2022). Low doses of the organic insecticide spinosad trigger lysosomal defects, elevated ROS, lipid dysregulation, and neurodegeneration in flies.Elife 11: e73812.
Competing interests
The author declares no conflicts of interest.
Ethics
Genetic modified Drosophila strain were maintained in a physical containment insectary facility following the biosafety guidelines by the Office of Research Ethics & Integrity at the University of Melbourne.
References
- Bell, H. S. and Tower, J. (2021). In vivo assay and modelling of protein and mitochondrial turnover during aging. Fly (Austin) 15(1): 60-72.
- Brand, M. D. and Nicholls, D. G. (2011). Assessing mitochondrial dysfunction in cells. Biochem J 435(2): 297-312.
- Chan, D. C. (2020). Mitochondrial Dynamics and Its Involvement in Disease. Annu Rev Pathol 15: 235-259.
- Chu, Y., Park, J., Kim, E. and Lee, S. (2021). Fluorescent Materials for Monitoring Mitochondrial Biology. Materials (Basel) 14(15): 4180.
- Denecke, S., Nowell, C. J., Fournier-Level, A., Perry, T. and Batterham, P. (2015). The Wiggle Index: An Open Source Bioassay to Assess Sub-Lethal Insecticide Response in Drosophila melanogaster. PLoS One 10(12): e0145051.
- Duranova, H., Valkova, V., Knazicka, Z., Olexikova, L. and Vasicek, J. (2020). Mitochondria: A worthwhile object for ultrastructural qualitative characterization and quantification of cells at physiological and pathophysiological states using conventional transmission electron microscopy. Acta Histochem 122(8): 151646.
- Ferree, A. W., Trudeau, K., Zik, E., Benador, I. Y., Twig, G., Gottlieb, R. A. and Shirihai, O. S. (2013). MitoTimer probe reveals the impact of autophagy, fusion, and motility on subcellular distribution of young and old mitochondrial protein and on relative mitochondrial protein age. Autophagy 9(11): 1887-1896.
- Filograna, R., Mennuni, M., Alsina, D. and Larsson, N. G. (2021). Mitochondrial DNA copy number in human disease: the more the better? FEBS Lett 595(8): 976-1002.
- Frenk, S. and Houseley, J. (2018). Gene expression hallmarks of cellular ageing. Biogerontology 19(6): 547-566.
- Gottlieb, R. A. and Stotland, A. (2015). MitoTimer: a novel protein for monitoring mitochondrial turnover in the heart. J Mol Med (Berl) 93(3): 271-278.
- Hernandez, G., Thornton, C., Stotland, A., Lui, D., Sin, J., Ramil, J., Magee, N., Andres, A., Quarato, G., Carreira, R. S., et al. (2013). MitoTimer: a novel tool for monitoring mitochondrial turnover. Autophagy 9(11): 1852-1861.
- Islam, M. T. (2017). Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 39(1): 73-82.
- Johnson, J., Mercado-Ayon, E., Mercado-Ayon, Y., Dong, Y. N., Halawani, S., Ngaba, L. and Lynch, D. R. (2021). Mitochondrial dysfunction in the development and progression of neurodegenerative diseases. Arch Biochem Biophys 702: 108698.
- Laker, R. C., Xu, P., Ryall, K. A., Sujkowski, A., Kenwood, B. M., Chain, K. H., Zhang, M., Royal, M. A., Hoehn, K. L., Driscoll, M., et al. (2014). A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J Biol Chem 289(17): 12005-12015.
- Landis, G. N., Abdueva, D., Skvortsov, D., Yang, J., Rabin, B. E., Carrick, J., Tavare, S. and Tower, J. (2004). Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci U S A 101(20): 7663-7668.
- Leboutet, R., Chen, Y., Legouis, R. and Culetto, E. (2020). Mitophagy during development and stress in C. elegans. Mech Ageing Dev 189: 111266.
- Liu, L., Zhang, K., Sandoval, H., Yamamoto, S., Jaiswal, M., Sanz, E., Li, Z., Hui, J., Graham, B. H., Quintana, A., et al. (2015). Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 160(1-2): 177-190.
- Martelli, F., Hernandes, N. H., Zuo, Z., Wang, J., Wong, C. O., Karagas, N. E., Roessner, U., Rupasinghe, T., Robin, C., Venkatachalam, K., et al. (2022). Low doses of the organic insecticide spinosad trigger lysosomal defects, elevated ROS, lipid dysregulation, and neurodegeneration in flies. Elife 11: e73812.
- Martelli, F., Zhongyuan, Z., Wang, J., Wong, C. O., Karagas, N. E., Roessner, U., Rupasinghe, T., Venkatachalam, K., Perry, T., Bellen, H. J., et al. (2020). Low doses of the neonicotinoid insecticide imidacloprid induce ROS triggering neurological and metabolic impairments in Drosophila. Proc Natl Acad Sci U S A 117(41): 25840-25850.
- Nichols, C. D., Becnel, J. and Pandey, U. B. (2012). Methods to assay Drosophila behavior. J Vis Exp (61): 3795.
- Nunnari, J. and Suomalainen, A. (2012). Mitochondria: in sickness and in health. Cell 148(6): 1145-1159.
- Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D. and Bitto, A. (2017). Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev 2017: 8416763.
- Shadel, G. S. and Horvath, T. L. (2015). Mitochondrial ROS signaling in organismal homeostasis. Cell 163(3): 560-569.
- Smolina, N., Bruton, J., Kostareva, A. and Sejersen, T. (2017). Assaying Mitochondrial Respiration as an Indicator of Cellular Metabolism and Fitness. Methods Mol Biol 1601: 79-87.
- Valko, M., Leibfritz, D., Moncol, J., Cronin, M. T., Mazur, M. and Telser, J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1): 44-84.
- Yan, L. J., Levine, R. L. and Sohal, R. S. (1997). Oxidative damage during aging targets mitochondrial aconitase. Proc Natl Acad Sci U S A 94(21): 11168-11172.
Article Information
Copyright
Martelli. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Martelli, F. (2022). Evaluation of Mitochondrial Turnover Using Fluorescence Microscopy in Drosophila. Bio-protocol 12(17): e4498. DOI: 10.21769/BioProtoc.4498.
- Martelli, F., Hernandes, N. H., Zuo, Z., Wang, J., Wong, C. O., Karagas, N. E., Roessner, U., Rupasinghe, T., Robin, C., Venkatachalam, K., et al. (2022). Low doses of the organic insecticide spinosad trigger lysosomal defects, elevated ROS, lipid dysregulation, and neurodegeneration in flies.Elife 11: e73812.
Category
Neuroscience > Cellular mechanisms > Mitochondria
Cell Biology > Cell imaging > Fluorescence
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









