- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Von Willebrand Factor Multimer Analysis by Low Resolution SDS-Agarose Gel Electrophoresis
Published: Vol 12, Iss 16, Aug 20, 2022 DOI: 10.21769/BioProtoc.4495 Views: 3314
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
A Novel Method to Make Polyacrylamide Gels with Mechanical Properties Resembling those of Biological Tissues
Katarzyna Pogoda [...] Paul A. Janmey
Aug 20, 2021 5568 Views
Evaluation of Urine Proteins by Capillary Electrophoresis
Paula F. Navarro [...] Salceda Fernández-Barredo
Aug 5, 2022 2155 Views
Reliable and Sensitive Detection of Carbonylated Proteins by Oxime Blot
Filip Luka Mikulić [...] Mladen Merćep
Aug 5, 2025 1233 Views
Abstract
Von Willebrand factor (VWF) is a complex glycoprotein found in plasma, composed of disulfide-bond-linked multimers with apparent molecular weights between 500 kDa and 20,000 kDa. After release of VWF from storage granules, it is cleaved in flowing blood by the specific metalloproteinase ADAMTS13, resulting in a highly characteristic cleavage pattern and structure. As the structure of VWF multimers determines diagnosis of von Willebrand disease, which has different sub-types with different multimer- and cleavage patterns, VWF analysis is performed using low-resolution horizontal SDS-agarose gel electrophoresis. However, almost every laboratory uses a different protocol, and all experimental details are rarely, if at all, described. Therefore, the results from similar methods may be substantially different. Here, we present a detailed description of a validated VWF multimer method that we have developed. It has been successfully used for over more than 20 years in quality control of recombinant and plasma-derived VWF drug products, and in preclinical and clinical studies with VWF drug candidates. As most of the published methods, it enables visualization of VWF multimers separated by electrophoresis by immunostaining with a polyclonal anti-human VWF antibody followed by a secondary antibody coupled to alkaline phosphatase. VWF appears as a series of regularly spaced bands on the low and middle molecular weight range of the gel, with an unresolved zone in the high molecular weight (HMW) range, where ultra-large multimers are found. An example is shown below. This low-resolution agarose gel electrophoresis allows the determination of the number of VWF multimers with high reproducibility.
Graphical abstract:

Example of electrophoretic analysis of multimer structure of four batches of a recombinant VWF drug substance.
Background
Von Willebrand factor (VWF) is the largest soluble plasma protein, with essential functions in blood coagulation, control of bleeding, and angiogenesis. It is a complex glycoprotein with a mature subunit of 2,050 amino acids (Turecek et al., 2017). Approximately 19% of its molecular size are N- and O-linked carbohydrates, some of which resembling important blood group antigens. VWF is synthesized in endothelial cells and megakaryocytes. Intracellular polymerization starts with the formation of disulfide bridged dimers, which subsequently assemble into multimeric structures via additional disulfide bonds, creating multimers with apparent molecular masses between 500 kDa and 20,000 kDa. VWF is released into the circulation from its storage granules as ultra-large VWF with ultra-high molecular weight (UHMW). These UHMW multimers are potent mediators of platelet adhesion to other platelets and to subendothelial structures exposed upon vessel damage. However, under normal circumstances, UHMW VWF multimers are rapidly cleaved by the plasma protease ADAMTS13 (A disintegrin-like and metalloprotease with thrombospondin type 1 repeats) to smaller sized multimers with lower hemostatic and, thus, lower thrombogenic potential. ADAMTS13 specifically cleaves VWF at the bond between Tyr1605 and Met1606, generating 140-kDa N-terminal and 176-kDa C-terminal fragments, thus, regulating multimer size. The number of VWF multimers is generally seen as an indicator of functionality because more binding sites (e.g., for collagen and platelets) are exposed from larger multimers when the molecules become elongated under shear stress in the vasculature (Favaloro et al., 2021).
The main function of VWF is to mediate platelet binding and aggregation at sites of vascular injury. This process is known as primary hemostasis. VWF is critical for the formation of the primary hemostatic platelet plug by serving as glue between platelets through binding to platelet receptors glycoprotein (GP) Ib-IX-V and αIIbβ3 integrin, in promoting primary platelet adhesion and aggregation following vessel injury, and promoting binding of platelet aggregates to subendothelial matrix proteins such as collagens, which become exposed to flowing blood when lesions occur in vessels (Bryckaert et al., 2015). Shear forces in blood vessels promote folding and unfolding of VWF, essentially regulating its binding properties to receptors, which are further controlled by limited proteolytic cleavage by ADAMTS13. Furthermore, VWF serves as chaperone protein for coagulation factor VIII (FVIII) through tight binding, maintaining a highly controlled equilibrium between free and bound FVIII, which is also dependent on multimer size. Additionally, VWF determines the circulatory half-life of FVIII. All these functions of VWF depend on multimer size, and the degree of VWF multimerization correlates with the ability to promote platelet aggregation, as an essential step in blood coagulation.
Defects in VWF and ADAMTS13 are involved in hemostatic and thrombotic disorders. Deficiencies in VWF result in von Willebrand disease (VWD). VWD is the most common inherited bleeding disorder in the world, with an estimated prevalence of symptomatic disease of approximately 1 in 1000 individuals. VWD is a heterogeneous disease with different genetic subtypes, called type 1, type 2 (with subtypes 2A, 2B, 2M, and 2N), and type 3 (Peyvandi et al., 2019). In addition to genetic analysis, differential diagnosis of VWD and its subtypes requires determination of VWF activity by different functional assays and determination of the multimer pattern. Phenotypic von VWD classification largely relies on analysis of multimeric distributions of VWF and evaluation of its structure. Examples of typical multimer patterns, as seen in VWF subtype patients, can be found in textbooks and review articles (e.g., Schneppenheim and Budde, 2011). Although VWF multimer analysis is labor intensive, non-standardized, and limited to specialized laboratories, efforts have been made recently to establish ranges for VWF multimer distribution (Vangenechten and Gadisseur, 2020).
Inherited or immune mediated deficiencies of ADAMTS13 impair VWF cleavage, resulting in an increased portion of ultra-large multimers, which can cause thrombosis in the microvasculature. This disease is called thrombotic thrombocytopenic purpura (TTP), and its differential diagnosis requires VWF multimer analysis. Most recently, an impairment of the ratio of VWF and ADAMTS13 was identified as the main determinant of severity of COVID-19, with highly elevated levels of VWF and reduced ADAMTS13 resulting in thrombosis of the microvasculature, particularly in the lungs (Turecek et al., 2021; Seth et al., 2022).
Since the development of the first electrophoretic methods to separate and visualize multimers of VWF, the principle of these methods has not changed (Ruggeri and Zimmerman, 1981). Despite some modest improvements, the quality of test results largely depends on the quality and concentration of the agarose gel and the equipment used. Thus, the most demanding diagnostic test for VWD is still the VWF multimer analysis, an investigation that challenges even the most seasoned laboratory technologist (Lillicrap, 2013). Therefore, it is a limitation that methods are often not fully described with all the experimental details required to make the analytical system robust and reproducible. While multimer analysis of VWF is now performed for more than 40 years (Meyer et al., 1980), only a few publications describe the experimental methods, and even these do not give precise indications on how to perform the analysis. Here, we describe a validated method for electrophoretic separation of VWF multimers in low resolution SDS-agarose gels, with subsequent visualization by immunostaining (Aihara et al., 1986), allowing densitometric analysis. The method equally qualifies for analysis of VWF in blood from humans and other species, diagnosis of VWD subtypes, and characterization of VWF preparations derived from human or animal blood, produced by expression in cell culture systems, or by vectors used in gene therapy (Turecek et al., 2010). This method has been successfully used in quality control of recombinant and plasma-derived VWF drug products, and in preclinical and clinical studies with VWF drug candidates (Turecek et al., 1997).
Materials and Reagents
Material
Flatbed electrophoresis apparatus and Multiphor II electrophoresis unit (with ceramic cooling plate), GE Healthcare, IL, USA (or equivalent)
Power Supply
Cryo thermostat, Modell MultiTemp III, (Cytiva, Uppsala, Sweden)
Hairdryer
Horizontal shaker
pH-meter
Vortex mixer
Water bath (up to 90 °C)
Pipettes and tips
Plastic test tubes 12 × 55 mm
Glass and plastic laboratory trays, approximately 14 × 26 × 6 cm
Glass plates, float glass, 11.5 × 23.0 × 0.2 cm (or equivalent)
Clamps for electrophoresis plates
Rubber seals for gel plates, U-shaped cut, approximately 1.5 mm thick
Rubber seals rectangular cut, 10 mm long, 4 mm wide, 1.5 mm thick
Disposable syringes, cannulas, and locking cone
GelbondFilm for agarose gel 124 × 258 mm (product: 80112932; Cytiva, Uppsala, Sweden)
Chromatography paper 1CHR (Whatman WHA1001917; Sigma, Vienna, Austria) and 3CHR (Whatman WHA3003917; Sigma, Vienna, Austria)
Paper towels/kitchen roll
Laboratory balances
Reagents
Purified water
Agarose SEAKEM HGT (prod. 50040; Lonza, Rockland, USA)
Agarose Biozym Plaque Agarose (prod. 840101; Biozym Scientific GmbH, Oldendorf, Germany)
Tris(hydroxymethyl-aminomethane)
Sodium dodecyl sulfate pellets (SDS)
Ethylen-diamin-tetraacetic acid-di-sodium salt EDTA
NaCl
Glycine
Bromophenol-blue
Triton X100
Na2HPO4·2H2O
NaH2PO4·H2O
Antibody rabbit anti-human-VWF (product A0082 DAKO, Agilent Technologies, Glostrup, Denmark)
Secondary antibody goat-anti-rabbit-IgG-ALP-conjugated. (product 111-055-003; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA)
AP Conjugate Substrate Kit (product 1706432, BioRad Laboratories, Hercules, CA, USA)
Human normal plasma pool (e.g., Precision Biologics, Rockville, USA)
0.9% NaCl
Separation gel buffer (see Recipes)
Stacking gel buffer (see Recipes)
Sample buffer (see Recipes)
Running buffer for electrophoresis apparatus (see Recipes)
Bromophenol blue solution (see Recipes)
PBS-Buffer for immuno-staining (in gel staining) (see Recipes)
Washing buffer/antibody dilution buffer (see Recipes)
Equipment
Densitometer GS-900 (BioRad Laboratories, Hercules, CA, USA)
Software Image Lab (BioRad Laboratories, Hercules, CA, USA) and/or Image Quant TL (Cytiva, Uppsala, Sweden)
Multiphor II Electrophoresis-System with cooling unit (Figure 1).
Microwave oven

Figure 1. Electrophoresis system: Cooling unit (left) and flatbed apparatus (right)
Procedure
Prepare the glass cassette for hand-made agarose gel
On a plane glass plate, glue rectangular rubber seals (10 mm long; 4 mm wide; 1.5 mm thick) on the long side of the glass plate (1.5 cm under the longitudinal edge)—14 pieces at regularly spaced distance. These will form the loading wells for samples (Figure 2A).
Place a separate plane glass plate on the bench (11.5 × 23.0 × 0.2 cm). Cut gelbond film to a length of 23 cm. With a few drops of purified water, fix the gelbond film with its hydrophobic side on the glass plate, avoiding air bubbles (Figure 2B).
Wet the U-shaped rubber seal and place it on the gelbond film (Figure 2C). Place the other glass plate with the loading rubber seals on top, so it forms a casting mold (cassette) (Figure 2D).
Fix the glass cassette with clamps (Figure 2E).
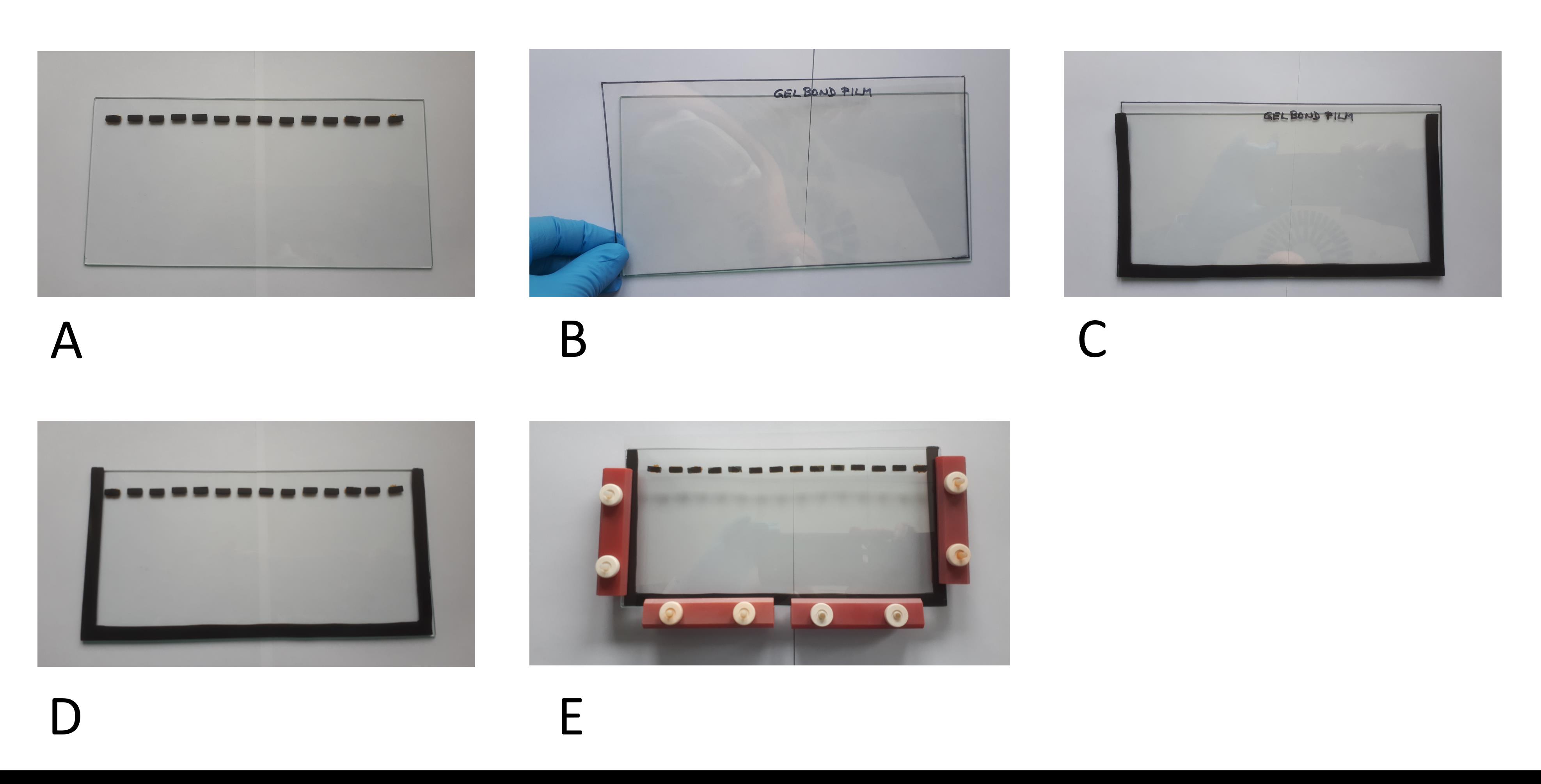
Figure 2. Preparation of the gel cassette.
Casting of separation gel
Caution: Hot liquids, handle with care. Wear protective equipment to prevent injury.
Prepare a 1% agarose gel. Suspend 0.5 g agarose SEAKEM HGT with 50 mL of separation gel buffer and heat the solution in a microwave oven (e.g., 900 W; interrupt heating when liquid starts to foam, remove from oven, and gently agitate; repeat procedure until complete dissolution). Draw this solution into a 50 mL syringe, close the syringe with a cone, and incubate it in a water bath at approximately 90 °C for approximately 30 min.
Place the glass cassette upright in a pre-warmed water bath at 60 ± 5 °C. To cast the gel, place a cannula on the syringe and slowly fill the hot agarose into the glass cassette (on one upper side of the glass cassette) until approximately 1 cm below the loading wells.
Take the glass cassette out of the water bath and allow the gel to set. Keep at room temperature (RT) for approximately 1 h, standing upright.
Casting of stacking gel
Caution: Hot liquids, handle with care. Wear protective equipment to prevent injury.
Suspend 0.2 g agarose SEAKEM HGT with 25 mL of stacking gel buffer in the microwave oven until complete dissolution. Cool to approximately 60–70 °C, and pipette the agarose on top of the separation gel until reaching the upper end of the cassette.
Once the cassette is fully filled, keep a small volume of stacking gel for Procedure F.
Allow the gel to set for approximately 1 h at RT. Open the glass cassette by carefully removing the clamps and the upper glass plate. Take out the gelbond film with the adhesive agarose gel.
Preparing the electrophoresis apparatus
Turn on the cryostat to cool down the flatbed ceramic plate at approximately 10 °C.
Add the running buffer (approximately 1 L per flatbed chamber) to the reservoirs at each end of the gel chamber.
Pipette a few drops of running buffer on the ceramic plate, and place on top the gelbond film with the gel, with the loading wells on the cathodic side.
Filter papers (1CHR) soaked with running buffer are placed as follows: five layers of paper (23 cm × 15 cm), one end in the cathodic buffer vessel, the other end attached to the cathodic end of the gel (do not overlap the gel), and on top another five layers slightly overlapping the agarose gel (keep at approximately 1 cm distance to the sample application wells). Repeat the same procedure on the anodic end of the gel, with five layers of 23 cm × 15 cm paper and five layers of 23 cm × 23 cm paper.
Sample preparation/loading
Caution: Hot liquids, handle with care. Wear protective equipment to prevent injury.
Dilute samples and assay control to the same VWF concentration (preferably 1 IU VWF:Ag/mL), using 0.9% NaCl as diluent.
Mix 0.2 g Biozym Plaque Agarose with 10 mL of sample buffer, and heat in the microwave oven until completely dissolved. Cool down in water bath to approximately 60 °C.
Mix 50 µL of diluted sample, 50 µL of sample buffer, 10 µL of bromophenol blue solution, and 100 µL of Biozym Plaque Agarose.
Incubate at 60 ± 5 °C for 20 ± 5 min.
Load a volume of 50 µL per loading well.
Electrophoresis
Close the lid and start the electrophoresis.
Running time: 15–20 h.
Running conditions: 25 mA constant for 1–3 h, until samples are fully run out of the wells. Interrupt the run to fill the empty wells with stacking gel (30 µL). Continue the separation with 8–10 mA for 14–16 h, until the bromophenol blue line has reached the end of the gel.
After separation, VWF multimers are visualized by either direct in-gel staining or after blot transfer. The protocol below describes the in-gel procedure only.
In gel immuno-staining
Drying the gelAfter finishing the gel run, remove the filter papers and wash the gel with purified water on a horizontal shaker for approximately 1–3 h, changing the water for a minimum of five times. The bromophenol blue line should no longer be visible.
Press the gel for 45 min: Place two chromatography paper sheets (3CHR) soaked with purified water on the gel, two more dry papers, and five more kitchen towels. Place one glass plate on top, and press with approximately 2 kg (e.g., place two 1 L bottles with water on top).
After pressing, remove all paper sheets, fix the gel on the bench, and carefully dry the gel with a hair dryer for approximately 30 min.
Immuno-staining
Note: The following procedure is for detection of human VWF. For visualization of VWF from other species, appropriate antibodies need to be selected.
Primary antibody: rabbit anti-human-VWF (A0082)
Place the gel in a tray.
The antibody is diluted to 1 µg/mL in washing buffer/antibody dilution buffer. Prepare a solution of 100 mL per gel, and add to the tray.
Incubate the gel under gentle agitation (shaker) for approximately 16 h.
Pour off the antibody solution and wash the gel with washing buffer/antibody dilution buffer for 5 h.
Secondary antibody: goat-anti-rabbit-IgG-ALP-conjugated
Dilute the secondary antibody with washing buffer/antibody dilution buffer to 0.6 µg/mL (100 mL per gel).
Place the gel into the diluted secondary antibody and incubate under gentle agitation for approximately 16 h.
Pour off the secondary antibody and wash the gel for 1 h in washing buffer/antibody dilution buffer.
Repeat the washing procedure for a further 3–4 h in PBS buffer for immunostaining (without Triton X100), by changing the buffer a minimum of five times.
Immuno-staining according to package insert from AP-Kit (Bio-Rad)
Prepare a solution with 4 mL of kit buffer, 1 mL of reagent A, and 1 mL of reagent B from the AP-Kit. Fill up to 100 mL with purified water.
Use a fresh tray. Place the gel in the staining solution and incubate on a shaker at RT for approximately 10–30 min. The incubation time depends on the band intensity. The bands must be clearly visible, without significant background stain.
Place the gel in purified water to stop staining. Wash with purified water for approximately 20 min.
Fix the gel horizontally on the sides with adhesive strips, and dry the gel either by air drying or with a hair dryer.
Data analysis
Gels can be analyzed visually through side-by-side analysis—the number of multimers, countable with the naked eye, can be recorded (Figure 3) or by densitometry (Figure 4).
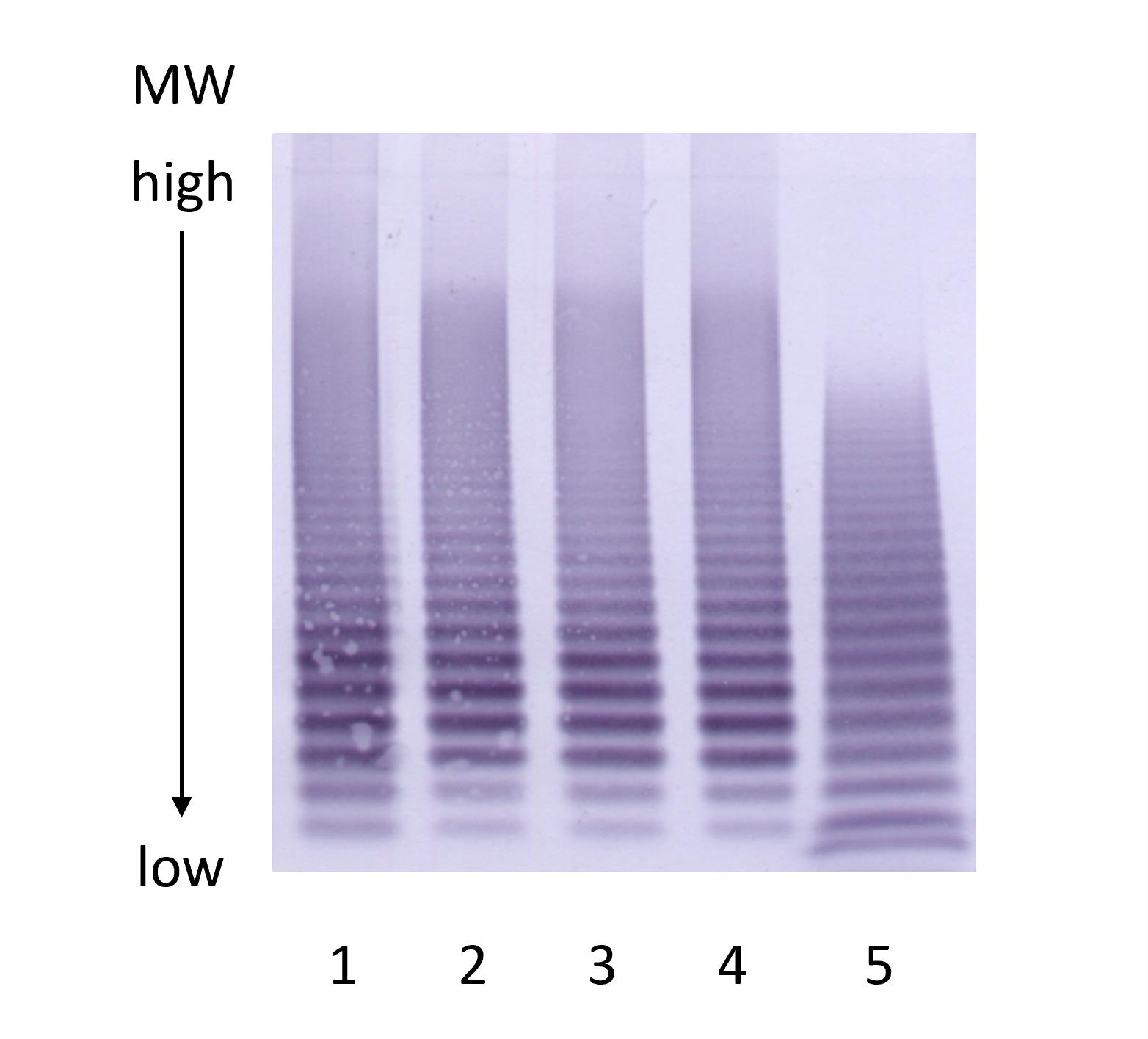
Figure 3. VWF multimer gel. Multimer gel. Lanes 1–4: recombinant VWF. Lane 5: normal human plasma pool.
For densitometric analysis, the gels are scanned with a densitometer (GS 900) and analyzed with an appropriate software (for example, Image Lab or Image Quant TL).
For each lane, the distance between the top of the separation gel and the lowest multimer band (corresponding to VWF dimers) is assigned a migration value of 1.0. The distance of the largest VWF multimer band in the sample lane is then measured and expressed as a fraction of this, denoted relative migration distance (Rf). The proportion of the total migration distance of the VWF dimer band that is occupied by all of the other multimers in the sample lane is therefore 1-Rf (Figure 4).
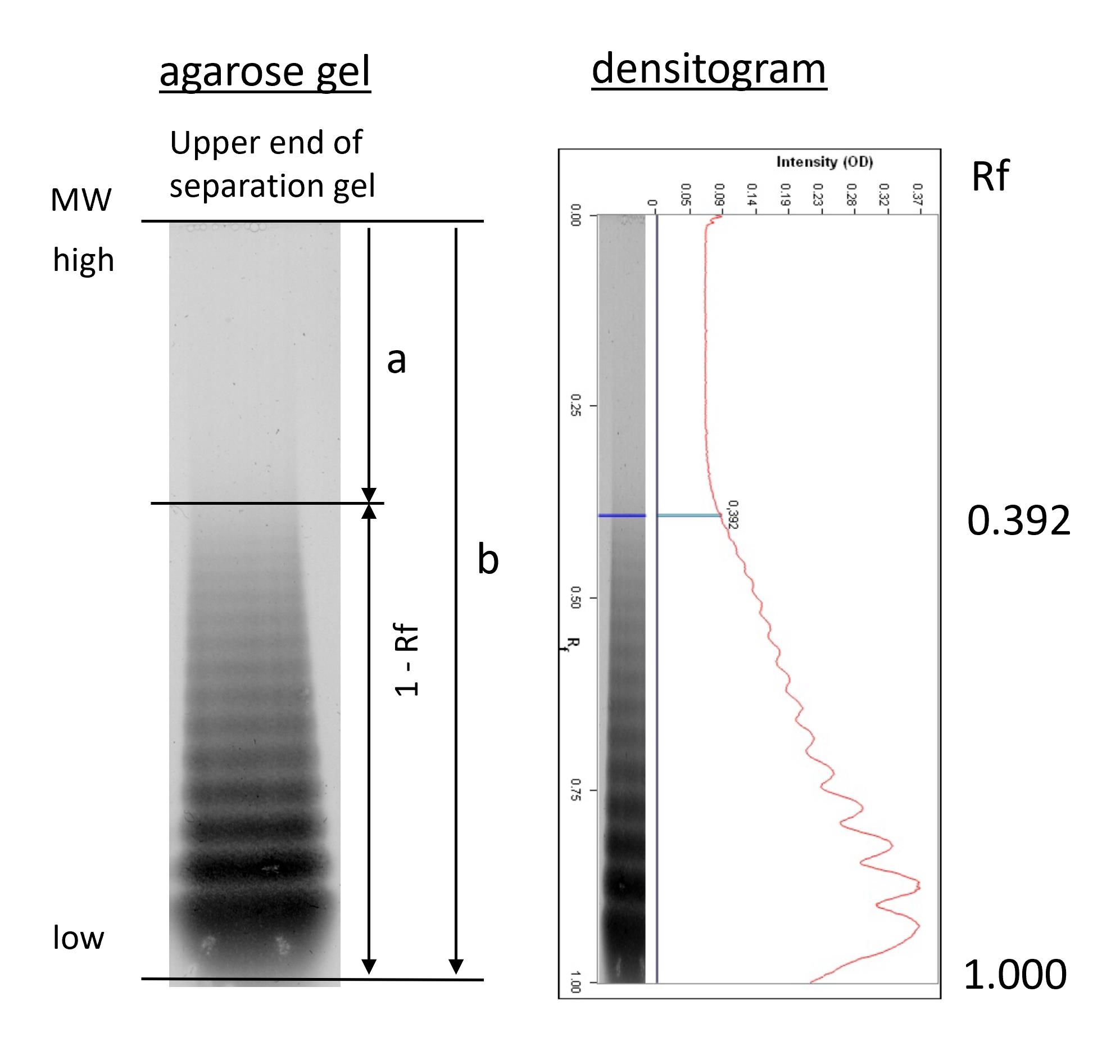
Figure 4. Densitometric analysis (details are described in Turecek et al., 2021). VWF: Ag concentration of the test sample was adjusted to 1 IU/ mL and separated on a 1% agarose gel, followed by immunostaining and densitometry. Multimer pattern (left) and densitogram (right). “a” is the migration distance of the largest VWF multimer, “b” is the migration distance of the smallest VWF (dimer), assigned a migration value of 1.000.
To enable a comparison between different electrophoresis gel runs, this value is reported as a percentage of the 1-Rf value for a normal plasma sample separated on the same gel. An increased percentage value indicates the presence of UHMW VWF multimers in the test sample.
In this example, the relative migration distance (Rf) of the largest VWF multimer was 0.392. The proportion of the separation lane that is occupied by VWF multimers (smallest to largest) is therefore 1-Rf, in this example, calculated as 1.000 – 0.392 = 0.608. The 1-Rf value for the test sample may be calculated as a percentage of the 1-Rf for a control sample run on the same gel to normalize results.
Recipes
Separation gel buffer
pH pH 8.8 ± 0.1 Ingredients 0.375 M Tris 0.1% SDS Preparation: Dissolve 45.43 g Tris and 1 g SDS with approximately 800 mL of purified water, adjust to pH 8.8 ± 0.1 with 25% HCl and fill up to 1,000 mL. Stacking gel buffer
pH pH 6.8 ± 0.1 Ingredients 0.125 M Tris 0.1% SDS Preparation: Dissolve 7.57 g Tris and 0.5 g SDS with approximately 400 mL of purified water, adjust to pH 6.8 ± 0.1 with 25% HCl and fill up to 500 mL. Sample buffer
pH pH 8.0 ± 0.1 Ingredients 0.01 M Tris 0.001 M EDTA 2% SDS Preparation: Dissolve 0.121 g Tris, 0.0372 g EDTA, and 2.0 g SDS with approximately 80 mL of purified water, adjust to pH 8.0 ± 0.1 with 25% HCl and fill up to 100 mL. Running buffer for electrophoresis apparatus
pH pH 8.4 ± 0.1 Ingredients 0.05 M Tris 0.384 M Glycine 0.1% SDS Preparation: Dissolve 12.11 g Tris, 57.65 g Glycine, and 2.0 g SDS with 2,000 mL of purified water. The pH value automatically reaches 8.4 ± 0.1. Bromophenol blue solution
Ingredients Bromophenol blue Sample buffer Preparation: Dissolve 0.1 g Bromophenol blue in 50 mL of sample buffer. Washing buffer/antibody dilution buffer
Ingredients Triton X100 PBS-buffer for immuno-staining Preparation: Dilute 10 g Triton X100 in 1,000 mL of PBS buffer for immuno-staining. PBS-Buffer for immuno-staining (in gel staining)
pH pH 7.4 ± 0.1 Ingredients 0.01 M Na2HPO4·2H2O 0.01 M NaH2PO4·H2O 0.154 M NaCl Preparation: Mix the two buffer solutions to reach pH 7.4 ± 0.1.
Preparation of PBS-solution secondary Phosphate:
Dissolve 17.8 g Na2HPO4·2H2O and 90 g NaCl in 10 L of purified water.
Preparation of PBS-solution primary Phosphate:
Dissolve 5.52 g NaH2PO4·H2O and 36 g NaCl in 4 L of purified water.
Dilute approximately 5 L of PBS-solution secondary Phosphate with PBS-solution primary Phosphate to reach pH 7.4 ± 0.1.Washing buffer/antibody dilution buffer
Ingredients Triton X100 PBS-buffer for immuno-staining Preparation: Dilute 10 g Triton X100 in 1,000 mL of PBS buffer for immuno-staining.
Acknowledgments
The basic protocol has been implemented in our laboratory in the mid 1980s by Fritz Elsinger. Since then, we are permanently pursuing VWF multimer analysis using the described electrophoresis method. Over the years the protocol was consistently optimized by subtle improvements. These had been elaborated in collaboration with many technicians working on the method. We acknowledge expert technical support from Alena Kratochwil, Ingrid Neunteufl, Gertrude Maurer, Gabriele Müllner, Nina Pruckner, Astrid Bogat, and Julia Lasnik.
Competing interests
HG, PLT and MS are employees of Baxalta Innovations GmbH, a Takeda company, PLT holds relevant Takeda patents and Takeda stock.
References
- Aihara, M., Sawada, Y., Ueno, K., Morimoto, S., Yoshida, Y., de Serres, M., Cooper, H. A. and Wagner, R. H. (1986). Visualization of von Willebrand factor multimers by immunoenzymatic stain using avidin-biotin peroxidase complex. Thromb Haemost 55(2): 263-267.
- Bryckaert, M., Rosa, J. P., Denis, C. V. and Lenting, P. J. (2015). Of von Willebrand factor and platelets. Cell Mol Life Sci 72(2): 307-326.
- Favaloro, E. J., Pasalic, L., Henry, B. and Lippi, G. (2021). Laboratory testing for ADAMTS13: Utility for TTP diagnosis/exclusion and beyond. Am J Hematol 96(8): 1049-1055.
- Lillicrap, D. (2013). von Willebrand disease: advances in pathogenetic understanding, diagnosis, and therapy. Blood 122(23): 3735-3740.
- Meyer, D., Obert, B., Pietu, G., Lavergne, J. M. and Zimmerman, T. S. (1980). Multimeric structure of factor VIII/von Willebrand factor in von Willebrand's disease. J Lab Clin Med 95(4): 590-602.
- Peyvandi, F., Kouides, P., Turecek, P. L., Dow, E. and Berntorp, E. (2019). Evolution of replacement therapy for von Willebrand disease: From plasma fraction to recombinant von Willebrand factor. Blood Rev 38: 100572.
- Ruggeri, Z. M. and Zimmerman, T. S. (1981). The complex multimeric composition of factor VIII/von Willebrand factor. Blood 57(6): 1140-1143.
- Seth, R., McKinnon, T. A. J. and Zhang, X. F. (2022). Contribution of the von Willebrand factor/ADAMTS13 imbalance to COVID-19 coagulopathy. Ame J Physiol Heart Circ Physiol 322(1): H87-H93.
- Schneppenheim, R. and Budde, U. (2011). von Willebrand factor: the complex molecular genetics of a multidomain and multifunctional protein. J Thromb Haemost.9, Suppl 1:209-15.
- Turecek, P. L., Gritsch, H., Pichler, L., Auer, W., Fischer, B., Mitterer, A., Mundt, W., Schlokat, U., Dorner, F., Brinkman, H. J., van Mourik, J. A. and Schwarz, H. P. (1997). In vivo characterization of recombinant von Willebrand factor in dogs with von Willebrand disease. Blood 90(9): 3555-3567.
- Turecek, P. L., Peck, R. C., Rangarajan, S., Reilly-Stitt, C., Laffan, M. A., Kazmi, R., James, I., Dushianthan, A., Schrenk, G., Gritsch, H., et al. (2021). Recombinant ADAMTS13 reduces abnormally up-regulated von Willebrand factor in plasma from patients with severe COVID-19. Thrombosis Research 201: 100-112.
- Turecek, P. L., Schrenk, G., Rottensteiner, H., Varadi, K., Bevers, E., Lenting, P., Ilk, N., Sleytr, U. B., Ehrlich, H. J. and Schwarz, H. P. (2010). Structure and function of a recombinant von Willebrand factor drug candidate. Semin Thromb Hemost 36(5): 510-521.
- Turecek, P. L., Spannagl, M., Kragh, T., Allmaier, G., Turecek, M., Schrenk, G., Gritsch, H., Obermann-Slupetzky, O., Ewenstein, B. M. and Valentino, L. A. (2017). The role of ultralarge multimers in recombinant human von Willebrand factor - a review of physico-and biochemical studies and findings in in vivo models and in humans with von Willebrand disease. Hamostaseologie 37(S 01): S15-S25.
- Vangenechten, I. and Gadisseur, A. (2020). Improving diagnosis of von Willebrand disease: Reference ranges for von Willebrand factor multimer distribution. Res Pract Thromb Haemost 4(6):1024-1034.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Gritsch, H., Stimpfl, M. and Turecek, P. L. (2022). Von Willebrand Factor Multimer Analysis by Low Resolution SDS-Agarose Gel Electrophoresis. Bio-protocol 12(16): e4495. DOI: 10.21769/BioProtoc.4495.
Category
Medicine > Cardiovascular system
Biochemistry > Protein > Electrophoresis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









