- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Immunohistochemistry of Immune Cells and Cells Bound to in vivo Administered Antibodies in Liver, Lung, Pancreas, and Colon of B6/lpr Mice
Published: Vol 12, Iss 14, Jul 20, 2022 DOI: 10.21769/BioProtoc.4468 Views: 3606
Reviewed by: Xiaoyi ZhengXiaoyu LiuEmilie Besnard

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2514 Views
PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3970 Views

Intraepidermal Nerve Fiber Quantification of the Mouse Hind Paw Footpads: A Detailed and Simplified Protocol
Anastasia Yerushkin [...] Amir Dori
Dec 5, 2025 1219 Views
Abstract
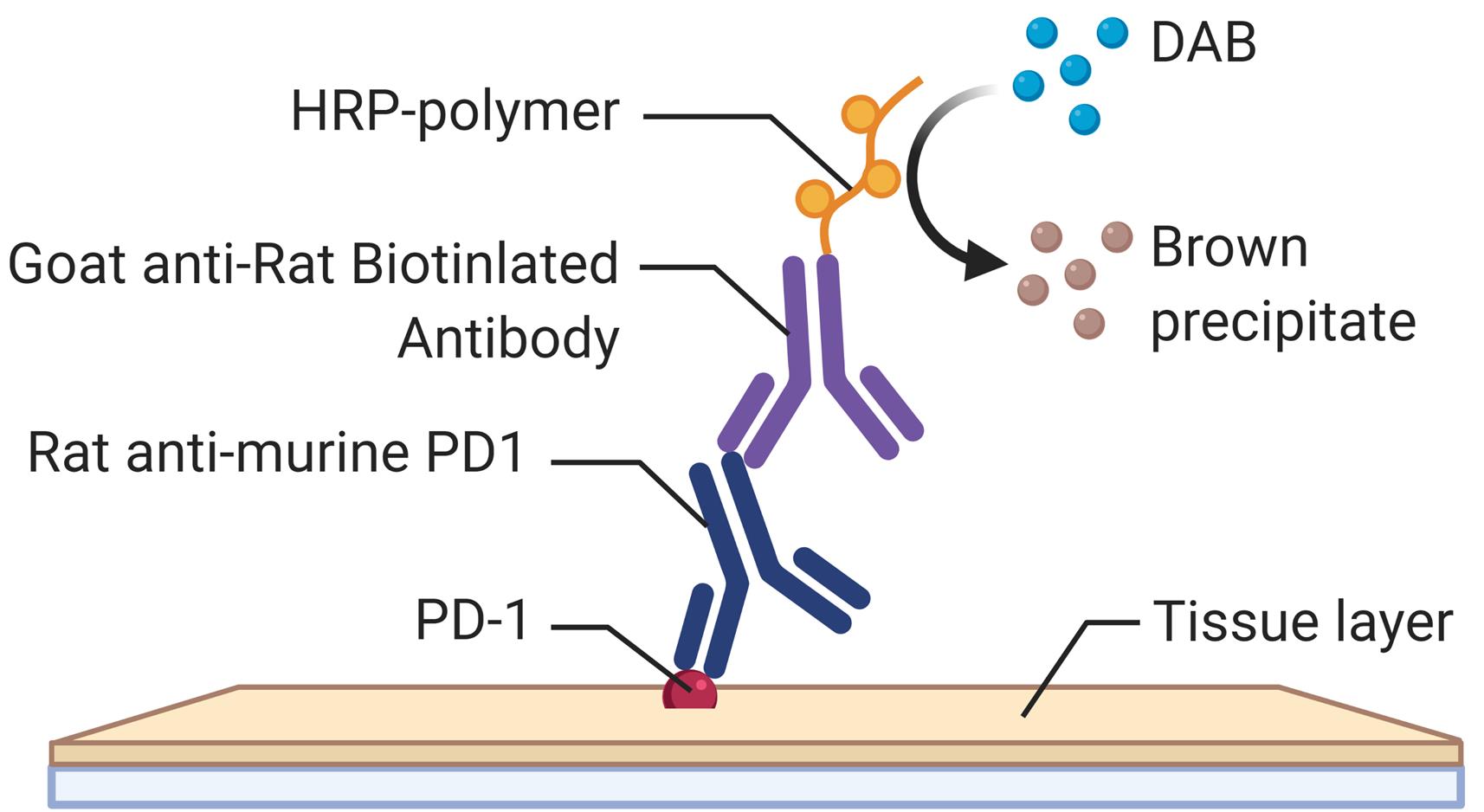
Employing a novel mouse model of immune related adverse events (irAEs) induced by combination of anti-PD1 and anti-CTLA-4 antibodies, we visualized immune infiltration into the liver, lung, pancreas, and colon. Here, we describe the avidin-biotin conjugate (ABC) method used to stain T cells (CD4 and CD8), B cells (CD19), macrophages (F4/80), and cells bound by the in vivo administered rat anti-mouse antibodies for chromogenic immunohistochemistry (IHC). Using a biotinylated goat anti-rat antibody, we detected the localization of cells bound to the in vivo antibodies for PD-1 and CTLA-4. IHC has advantages over other techniques, namely antibody availability, resistance to photobleaching, and greater sensitivity. Additionally, detection and localization of in vivo antibodies can be used in mice models to infer their therapeutic efficacy, stability, and function.
Graphical abstract:
Background
The last decade has seen the approval of several immune checkpoint blockade (ICB) antibodies in various cancers, which has improved patient survival. However, patients that respond to these ICB develop toxicities known as irAEs. We have previously developed a novel mouse model of irAEs in B6/lpr mice, that developed multiorgan toxicities with immune infiltration, when given a combination of anti-PD-1 and anti-CTLA-4 (Adam et al., 2021). Mice were injected with 200 μg of anti-PD1 (BioXCell BE0146), and 100 μg of CTLA-4 (BioXcell BE0131) antibodies intraperitoneally twice a week for 6 weeks. Mice were sacrificed at endpoint, and tissue collected for IHC, to assess the cellular landscape. The injected ICB antibodies derived from rat were visualized in the tissue using a goat anti-rat that recognized the fragment crystallizable (Fc) portion of the IgG rat antibody. This method of detection could be used to evaluate ICB antibodies for toxicity in mice models. Although there are numerous other techniques for visualization or detection of antibodies (de Boer et al., 2015; Mestel, 2017; Knight et al., 2019; Wen et al., 2021), the limitations are the access to large and expensive microscopes, CT scanner, or MRI, which may be inaccessible to many laboratories. Our method is advantageous due to a relatively low expense, accessible selection of conjugated antibodies, and flexibility with IHC kits.
Materials and Reagents
Tissue Tek Slide Staining Dish (Fisher, catalog number: NC0731403)
Slide Holder (Fisher, catalog number: NC9697666
10 mL pre-filled flush syringe saline (NaCl 0.9%) (BD, catalog number: 306575)
25G × ¾’’ blood collection set (BD, catalog number: 367285)
FisherbrandTM ColorFrostTM Plus Microscope Slides (Fisher, catalog number: 12-550-17)
FisherbrandTM Standard Disposable Transfer Pipettes, Nongraduated (Fisher, catalog number: 13-711-7M)
Goat anti-rat IgG antibody (H+L), biotinylated (Vector, catalog number: BA-9400)
Goat anti-rabbit IgG antibody (H+L), biotinylated (Vector, catalog number: BA-1000)
VECTASTAIN(r) Elite ABC-HRP Kit, peroxidase (Standard) (Vector, catalog number: PK-6100)
Dako liquid 3,3'-diaminobenzidine (DAB) and substrate (DAKO, catalog number: K3468)
CD4 (D7D2Z) (CST, catalog number: 25229)
CD8a (D4W2Z) (CST, catalog number: 98941)
CD19 (D4V4B) (CST, catalog number: 90176)
F4/80 (BM8) (Invitrogen, catalog number: 144801)
Xylene (Sigma, catalog number: 534056)
10× Citrate buffer pH 6.0 (Novus Biologicals, catalog number: NB900-62075)
Triton X-100 (Sigma, catalog number: X100)
Ethanol (Fisher Bioreagents, catalog number: BP2818100)
Hydrogen peroxide (BioRad, catalog number: BUF017B)
Hematoxylin counterstain (Vector, catalog number: H-3401-500)
Proteinase K (abcam, catalog number: ab64220)
10× PBS (Phosphate Buffered Saline) (Thermo Scientific, catalog number: AAJ75889K8)
Normal goat serum blocking solution (Vector, catalog number: S-1000-20)
FisherbrandTM SuperfrostTM ExcellTM Microscope Slides (Fisher, catalog number: 22-037-247)
Fisher ChemicalTM PermountTM Mounting Medium (Fisher, catalog number: SP15-100)
Dako Antibody Diluent (Agilent, catalog number: S0809)
Deionized water
B6/lpr (Jackson Laboratory, catalog number: 000482)
10% Neutral Buffered Formalin (Fisher, catalog number: 22-110-869)
Globe Scientific Rectangular Cover Glass size 24 × 50 mm, No.1 (Fisher, catalog number: 22-170-388)
Tween 20 (Sigma, catalog number: P1379)
PBS (Phosphate Buffered Saline) and PBS-T (Tween) (see Recipes)
10 mM Sodium Citrate (see Recipes)
Bluing solution (see Recipes)
Equipment
Forceps
Microtome (any supplier)
Microwave (any supplier)
Pressure cooker (any supplier)
Leica SCN400 scanner
Fume hood (any supplier)
Light microscope with 20× and 40× lenses
Water bath (ISOtemp205)
Fisher Scientific Isotemp 650D incubator oven
Software
AperioImage Scope (Leica, https://www.leicabiosystems.com/us/digital-pathology/manage/aperio-imagescope)
Procedure
Tissue processing (liver, lung, pancreas, and colon)
Collect tissue
Euthanize mice with CO2 and cervical dislocation.
Incise the skin from chin to thorax.
Cut away the sternum.
Using a 25G butterfly needle attached to a 10 mL syringe with 0.9% saline, insert this into the left ventricle, near the apex of the heart.
Cut the right atrium, and perfuse with saline.
Collect liver, lung, pancreas, and colon.
Fix tissue
Use 10 mL of 10% formalin for colon and pancreas, and 20 mL of 10% formalin for liver and lung.
Fix at room temperature for 48 h.
Dehydrate tissue
Immerse tissue in 70% ethanol at room temperature for 15 min.
Immerse tissue in 90% ethanol at room temperature for 15 min.
Immerse tissue in 100% ethanol at room temperature three times for 30 min each.
Immerse tissue in xylene at room temperature three times for 20 min each.
Heat paraffin in Isotemp incubator oven.
Embed tissue in paraffin at 58°C. As the paraffin will take several minutes to harden, this can be a pause point in the procedure.
Cut 5 μm-thick sections using a microtome.
Place each section in a water bath, so that it floats, and scoop with a slide to mount the section.
Dry slides at room temperature overnight.
Deparaffinization, and hydration
Immerse slides through the following solutions:
Xylene (pure), three washes of 3 min each.
100% Ethanol, two washes of 1 min each.
95% Ethanol, two washes of 1 min each.
70% Ethanol, one wash of 1 min.
Wash with deionized water two times for 3 min each.
Heat-induced antigen retrieval
Add 10 mM Sodium Citrate buffer (pH 6.0) to a staining dish with slides, and place into a pressure cooker or in microwave to bring to the boil for 10 min, using full power. Then, maintain at half power for 10 min (total of 20 min). For proteinase K retrieval, add 200 μL onto the section, incubate at room temperature for 5 min, and follow step C.4.
Allow to cool down at room temperature for 30 min.
Wash slides in distilled water for 5 min.
Wash slides in PBS-T three times for 5 min each.
Peroxidase quenching
To block endogenous peroxidase activity:
Quench section in 3.0% hydrogen peroxide in PBS-T for 10 min.
Wash slides in PBS-T three times for 5 min each.
Permeabilization and blocking of non-specific binding
Permeabilization
Wash sections in 0.1% Triton X-100 in PBS once for 10 min.
Wash slides in PBS-T three times for 5 min each.
Blocking
Block non-specific binding, by incubating the tissue section in 10% goat serum in PBS-T at room temperature for 25 min.
Antibody incubation
Primary antibody staining in Dako antibody diluent, as per dilutions in notes below.
Incubate at room temperature for 1.5 h.
Wash in PBS-T three times for 5 min each.
Secondary antibody staining.
Add biotinylated secondary antibody (1:200) and incubate at room temperature for 30 min.
Wash in PBS-T three times for 5 min each.
Detection
ABC (avidin/biotinylated enzyme complex) method:
Prepare working solutions of reagent A (avidin) and B (biotinylated HRP) in PBS at 1:50 dilution, 30 min prior. To prepare 1:50 dilution, add 100 μL of each reagent into 5 mL of PBS.
Incubate tissue sections at room temperature for 30 min.
Wash in PBS three times for 5 min each.
Prepare DAB (3,3'Diaminobenzidine)
Determine the volume of DAB needed (100 μL per section). Following the manufacturer’s instructions, add one drop of DAB per milliliter of substrate buffer.
Warning: Wear gloves, and work in the fume hood.
Add 100 μL of DAB working solution to each section. The biotinylated antibody with HRP will oxidize DAB and produce a brown precipitate at the site of the antigen. Monitor the slide every 5 min to visualize the brown color.
When the desired staining develops brown color, wash slides with deionized water.
Counterstaining
Counterstain using hematoxylin.
Immerse slides in hematoxylin to cover the tissue sections.
Incubate for 16 s.
Rinse section with tap water, until water is colorless.
Dip slides ten times in acid rinse solution, followed by ten dips in tap water.
Incubate slides in bluing solution.
Note: Hematoxylin is filtered once a week.
Slide dehydration, and mounting
Dehydrate slides by passing through the following solutions twice for 1 min each:
95% Ethanol
100% Ethanol
Xylene (pure)
Mount coverslips
Using a transfer pipette, add one drop of mounting media per slide and place the coverslip. Allow to dry for 1–2 h.
Slide visualization
Scan slides with a Leica SCN400, or use a microscope to visualize the tissue sections (Figure 1).
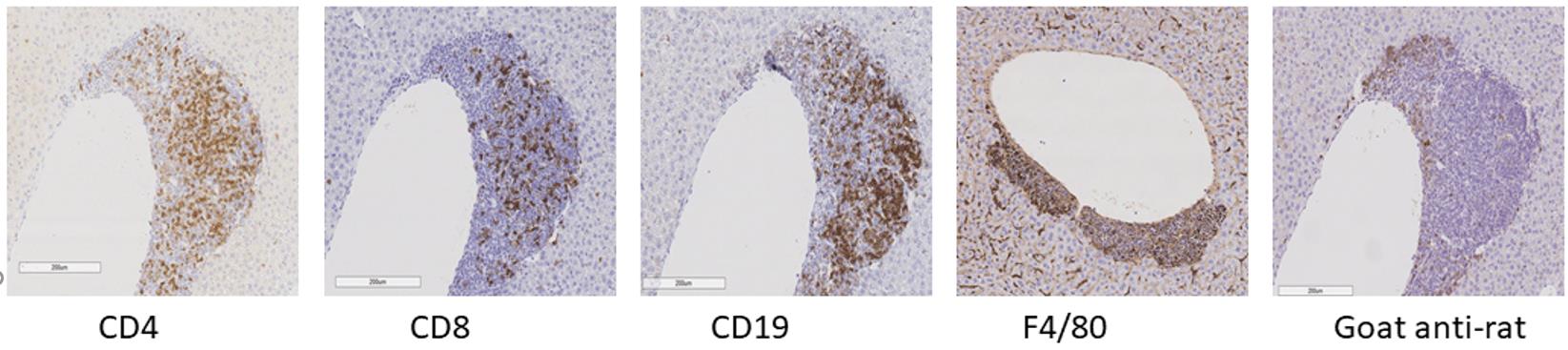
Figure 1. Immunohistochemistry of murine liver.
Notes
| Antibody name | Company | Cat # | Antigen retrieval | Antibody Dilution |
|---|---|---|---|---|
| CD19 | Cell Signaling | 90176 | citrate buffer | 1:1,000 (1 μL antibody + 999 μL diluent) |
| CD8a | Cell Signaling | 98941 | citrate buffer | 1:100 |
| CD4 | Cell Signaling | 25229 | citrate buffer | 1:80 |
| F4/80 | eBioscience | 14-4801 | proteinase K | 1:200 (5 μL antibody + 995 μL diluent) |
| Biotinylated goat anti rat | Vector | BA-9400 | citrate buffer | 1:200 |
Recipes
PBS (Phosphate Buffered Saline) and PBS-T (Tween)
1× PBS (900 mL of water + 100 mL of 10× PBS)
0.05% Tween 20 (add 0.5 mL of Tween 20 to 1 L of 1× PBS)
0.1% Triton (add 1 mL of Triton to 1 L of 1× PBS)
10 mM Sodium Citrate
10 mL of 10× Citrate + 90 mL of water
Bluing solution
1.5 mL of NH4OH (30% stock)
98.5 mL of 70% Ethanol
Acknowledgments
We would like to thank Shalom Lerrer, Sean Chen, Hongyan Tang, Tao Su, and Sun Dajiang for their support and expertise. This work was supported by grants from the NIH (AI125640, CA231277, AI150597) and the Cancer Research Institute. The method described here was used in the publication (Adam et al., 2021).
Competing interests
The author reports no competing interests.
Ethics
Animals used in accordance with NIH guidelines and approved by Columbia University’s Institutional Animal Use and Care Committee. Graphic was from BioRender stock IHC image. This protocol was adapted from: https://www.rndsystems.com/resources/protocols/protocol-preparation-and-chromogenic-ihc-staining-paraffin-embedded-tissue.
References
- Adam, K., Iuga, A., Tocheva, A. S. and Mor, A. (2021). A novel mouse model for checkpoint inhibitor-induced adverse events. PLoS One 16(2): e0246168.
- de Boer, E., Warram, J. M., Tucker, M. D., Hartman, Y. E., Moore, L. S., de Jong, J. S., Chung, T. K., Korb, M. L., Zinn, K. R., van Dam, G. M., et al. (2015). In Vivo Fluorescence Immunohistochemistry: Localization of Fluorescently Labeled Cetuximab in Squamous Cell Carcinomas. Sci Rep 5: 10169.
- Mestel, R. (2017). Cancer: Imaging with antibodies. Nature 543(7647): 743-746.
- Knight, J. C., Mosley, M. J., Kersemans, V., Dias, G. M., Allen, P. D., Smart, S. and Cornelissen, B. (2019). Dual-isotope imaging allows in vivo immunohistochemistry using radiolabelled antibodies in tumours. Nucl Med Biol 70: 14-22.
- Wen, L., Xia, L., Guo, X., Huang, H. F., Wang, F., Yang, X. T., Yang, Z. and Zhu, H. (2021). Multimodal Imaging Technology Effectively Monitors HER2 Expression in Tumors Using Trastuzumab-Coupled Organic Nanoparticles in Patient-Derived Xenograft Mice Models. Front Oncol 11: 778728.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Adam, K. and Mor, A. (2022). Immunohistochemistry of Immune Cells and Cells Bound to in vivo Administered Antibodies in Liver, Lung, Pancreas, and Colon of B6/lpr Mice. Bio-protocol 12(14): e4468. DOI: 10.21769/BioProtoc.4468.
Category
Immunology > Animal model > Mouse
Immunology > Immune cell staining > Immunodetection
Cell Biology > Cell staining > Protein
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








