- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Transient Middle Cerebral Artery Occlusion with an Intraluminal Suture Enables Reproducible Induction of Ischemic Stroke in Mice
(*contributed equally to this work) Published: Vol 12, Iss 3, Feb 5, 2022 DOI: 10.21769/BioProtoc.4305 Views: 7557
Reviewed by: Nafisa M. JadavjiMaria NguyenAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Intra-arterial Drug Delivery to the Ischemic Brain in Rat Middle Cerebral Artery Occlusion Model
Fereshteh Azedi [...] Mohammad Taghi Joghataei
Dec 5, 2019 7015 Views
Developing a Ministroke Model in Mouse Barrel Cortex
Song Wang [...] Yihan Wu
Mar 20, 2025 2306 Views
Utilizing EdU to Track Leukocyte Recruitment to the Brain
Zoie K. Lipfert [...] David P. Sullivan
Dec 5, 2025 1555 Views
Abstract
Ischemic stroke is a leading cause of mortality and chronic disability worldwide, underscoring the need for reliable and accurate animal models to study this disease’s pathology, molecular mechanisms of injury, and treatment approaches. As most clinical strokes occur in regions supplied by the middle cerebral artery (MCA), several experimental models have been developed to simulate an MCA occlusion (MCAO), including transcranial MCAO, micro- or macro-sphere embolism, thromboembolisation, photothrombosis, Endothelin-1 injection, and – the most common method for ischemic stroke induction in murine models – intraluminal MCAO. In the intraluminal MCAO model, the external carotid artery (ECA) is permanently ligated, after which a partially-coated monofilament is inserted and advanced proximally to the common carotid artery (CCA) bifurcation, before being introduced into the internal carotid artery (ICA). The coated tip of the monofilament is then advanced to the origin of the MCA and secured for the duration of occlusion. With respect to other MCAO models, this model offers enhanced reproducibility regarding infarct volume and cognitive/functional deficits, and does not require a craniotomy. Here, we provide a detailed protocol for the surgical induction of unilateral transient ischemic stroke in mice, using the intraluminal MCAO model.
Graphic abstract:

Overview of the intraluminal monofilament method for transient middle cerebral artery occlusion (MCAO) in mouse.
Background
With a global prevalence of more than 101.5 million, stroke is a leading cause of mortality and chronic disability worldwide (Benjamin et al., 2019; Diseases and Injuries, 2020). Though some strokes are caused when a vessel ruptures (i.e., hemorrhagic stroke), ischemic strokes account for 87% of clinical cases and occur following vascular occlusion, either by local arterial thrombus formation (e.g., cerebral atherosclerotic stroke) or a traveling embolic clot (e.g., cardioembolism) (Virani et al., 2021). Acute interventions to restore perfusion and halt neuronal death include pharmacological thrombolysis [i.e., intravenous recombinant tissue plasminogen activator (rtPA)] and mechanical endovascular thrombectomy (Pierot et al., 2018; Powers et al., 2018; Adams and Nudo, 2013), though clinical exclusion criteria restrict eligibility to just 20% of patients (National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995; Hacke et al., 2008; Saver et al., 2009; Smith and Furlan, 2016; Yaghi et al., 2017; Evans et al., 2017; Albers et al., 2018; Nogueira et al., 2018; Powers et al., 2018; Mokin et al., 2018).
The necessity for more inclusive therapeutic options underscores the significance of animal models, which have long been used to study disease pathology, identify molecular mechanisms of injury, and test treatment paradigms (Narayan et al., 2021). While large animal models (i.e., pigs, dogs, non-human primates) serve a key role, given their higher order resemblance to the human condition (Kaiser and West, 2020), small animals are most commonly used in preclinical stroke research. Factors specifically favoring murine models (i.e., mice and rats) include lower cost, reliable physiological responses, procedural reproducibility, available imaging modalities, a variety of functional and behavioral tests, and options for genetic manipulation (Fluri et al., 2015; Hermann et al., 2019).
The majority of clinical strokes occur in regions supplied by the middle cerebral artery (MCA), a terminal branch of the internal carotid artery (ICA) that supplies two-thirds of the lateral cortex, the infra-lateral surface of the anterior brain, and many deep subcortical structures (e.g., internal capsule and basal ganglia) (Nogles and Galuska, 2021). Numerous preclinical approaches to experimental MCA occlusion (MCAO) have been described, including direct suturing or electrocoagulation of the MCA (transcranial MCAO), intraluminal placement of a monofilament (intraluminal MCAO), delivery of synthetic (micro- or macro-sphere embolisation) or autologous clots (thromboembolisation), transcranial vessel illumination following photosensitive dye injection (photothrombosis), and stereotactic injection of a potent vasoconstrictor (Endothelin-1) (Fluri et al., 2015; Hermann et al., 2019; Kumar et al., 2016). Of these methods, intraluminal MCAO is the most common technique applied for ischemic stroke induction in murine models.
First introduced into mice in 1993 by Chan et al. (1993), the intraluminal MCAO procedure induces a proximal large vessel occlusion in mice, through placement of a coated monofilament tip at the MCA origin. The external carotid artery (ECA) is permanently ligated, after which the partially-coated monofilament is inserted and coursed proximally to the common carotid artery (CCA) bifurcation, before being introduced into the ICA. The coated tip of the monofilament is advanced to the origin of the MCA and secured for the duration of the occlusion. Reduction of blood flow into the MCA is confirmed using Laser Doppler Flowmetry (LDF) (40-70% reduction). Adjustable variables that alter stroke severity and distribution include monofilament composition (e.g., nylon) and diameter (e.g., 3-0), monofilament tip coating material (e.g., silicone), insertion length of the monofilament (e.g., 8 mm), duration of surgery (e.g., 45 min), and duration of occlusion (e.g., 30 min) (Engel et al., 2011; Fluri et al., 2015; Lee et al., 2014). Though this model allows for permanent occlusion of the MCA, transient MCAO (tMCAO) is achieved by retracting the monofilament tip to allow for reperfusion, and is thus more clinically relevant. Additional advantages of this model are its reproducibility, regarding both infarct volume and cognitive/functional deficits, and the fact that a craniotomy is not required. tMCAO also produces an ischemic core and penumbral region, which facilitates the study of neuronal death, neuroinflammation, and blood-brain-barrier breakdown, as well as testing of therapies to rescue the penumbra.
Our group has long used the intraluminal tMCAO model of ischemic stroke induction (Khanna et al., 2017; Rink et al., 2017; Sen et al., 2017; Balch et al., 2021; Lemmerman et al., 2021). Limitations to this model include larger infarction compared to human strokes and compromised hypothalamic regulation of body temperature, which is not typically present in humans and complicates survivability of murine models (Carmichael, 2005). Additionally, distal ligation of the ECA removes blood flow to masticatory muscles, which can further reduce eating in an animal facing significant weight loss (Dittmar et al., 2003). Despite these limitations, MCAO is well-established and widely accepted for murine ischemic stroke induction in preclinical research (Fluri et al., 2015). In conjunction with a dedicated small animal surgeon, our group has spent over seven years refining the tMCAO approach, to address the limitations related to food/water intake, weight loss, and mortality. Our refined protocol for surgery and post-operative care yields successful MCA-territory infarction, while enhancing reproducibility and animal survivability. Here, we provide a detailed protocol for surgical induction of unilateral transient ischemic stroke using our refined intraluminal thread model of transient unilateral MCAO.
Materials and Reagents
Silicone Rubber-Coated Monofilament (Doccol, catalog number: 602323PK10)
Note: Monofilament size should be selected considering the weight of mice used in the study. The specific catalog number provided is suitable for animals with body weight in the range 30 ± 5 grams, as per the manufacturer.
6-0 Spool Black Braided Silk Sutures (HOSPEQ, catalog number: sp114)
6-0, P-10 Braided Polyester Absorbable Sutures (Henry Schein, Polysorb, catalog number: 2946794)
6-0, C-22 Black Braided Silk Sutures (Henry Schein, catalog number: 101-2636)
Vet Bond Tissue Adhesive (Fisher Scientific, catalog number: 15672)
Surgical Tape (3M, catalog number: 1527-0)
Cotton Tip Applicator (McKesson, Q-Tip, catalog number: 785468)
Surgical Drapes (SAI Infusion Technologies, catalog number: PSS-SD5)
Note: To provide an increased view, the surgical drapes were removed prior to images being acquired for this protocol.
Isoflurane (Primal Health Care, catalog number: NDC 66794-017-10)
Artificial tear ointment (Henry Schein, catalog number: 1338333)
Chlorhexidine (Aspen, catalog number: NDC 46066-141-01)
0.9% sodium chloride solution (Saline) (Henry Shein, catalog number: 1047098)
Ethanol, 200 Proof (100%) (Decon Labs, catalog number: 2701)
70% ethanol solution (see Recipes)
Equipment
Dumont #4 forceps (Fine Science Tools, catalog number: 11294-00)
Dumont #5 forceps 45° Inox tip (Roboz, catalog number: RS-5005)
Graefe curved and serrated forceps (×2) (Fine Science Tools, catalog number: 11051-10)
Moria Iris forceps (×2) (Fine Science Tools, catalog number: 11370-31)
Extra Fine Bonn (Iris) Scissors (Fine Science Tools, catalog number: 14084-08)
Tenotomy scissors (Fine Science Tools, catalog number: 14066-11)
Vannas Spring scissors 3mm cutting edge (Fine Science Tools, catalog number: 15000-00)
AutoClip Applier (Fine Science Tools, catalog number: 12020-09)
AutoClips (Fine Science Tools, catalog number: 12022-09)
AutoClip Remover (Fine Science Tools, catalog number: 12023-00)
Castroviejo needle Holder-Locking (Roboz, catalog number: RS-6416)
Micro clip curved (Roboz, catalog number: RS-5433)
Micro clip straight (Roboz, catalog number: RS-5424)
Mirco clip applying forceps (Roboz, catalog number: RS-5410)
Bipolar Coagulator System (Accurate Surgical, Polarmate, catalog number: AC1905)
Bipolar Forceps (Accurate Surgical, Adson, catalog number: ASSI.BPNS12723)
Laser Doppler System (Moor Instruments, catalog number: MOORVMS-LDF1-HP)
Optic Fiber (Moor Instruments, catalog number: POF500)
Slide Warmer With Temperature Control (4MD Medical, catalog number: CASCXH-2001)
Somnosuite Low-Flow Anesthesia System (Kent Scientific, catalog number: n/a)
Induction Chamber (Kent Scientific, catalog number: Somno-0705)
Warming Pad (Kent Scientific, catalog number: RT-0501)
Electric Razor with Vacuum (Remmington, catalog number: VPG-6530)
Microscope with Foot Pedals (Leica, catalog number: Wild M691)
Procedure
Preparation
Sterilize all surgical instruments, surgical tray, and materials prior to surgery.
Note: Between same-day animal surgeries, soak instruments in 70% ethanol solution and rinse them with sterile water prior to the next surgery.
Set and maintain the surgical station warming pad at 37°C.
Place a new sterile cage on a slide warmer set to 37°C.
Note: As mice are singly-housed post-MCAO, one cage will be needed per mouse. This cage will serve as housing during the occluding event and again upon completion of the procedure.
Measure and mark the monofilament at distances of 6.5 mm and 10 mm from the tip of the coated end.
Note: This step delineates the acceptable range for insertion length of the silicone rubber-coated monofilament. For visualization, we recommend using a metallic Sharpie to mark the monofilament. Confirm that marks are dry prior to starting the procedure.
Weigh and record the baseline (i.e., pre-MCAO) weight of the mouse.
Note: Baseline and post-operative weight values can be compared to evaluate post-stroke weight loss (e.g., as a percentage of baseline), as an indicator of mouse recovery.
Surgery
Place the mouse in the induction chamber. Using the Somnosuite Low-Flow Anesthesia System, deliver a 4-5% mixture of isoflurane in room air at ~500 mL/min to the induction chamber, to anesthetize the mouse. After the mouse has reached anesthetic depth, move the mouse to the nose cone and warming pad. Set the Anesthesia System to deliver a 1-2% mixture of isoflurane in room air at ~250 mL/min to the nose cone for the duration of the procedure.
Note: Confirm anesthetic depth of the mouse by testing the toe-pinch reflex.
Apply artificial tear ointment to the eyes of the mouse.
Place the animal on its left side.
Prepare the area between the right eye and right ear for an incision by cleaning the area with chlorhexidine and parting the hair (Figure 1A).
Make an incision in the prepped area for the LDF optic fiber to be placed against the skull (Figure 1B).
Note: Do not place the LDF optic fiber against the skull at this step.
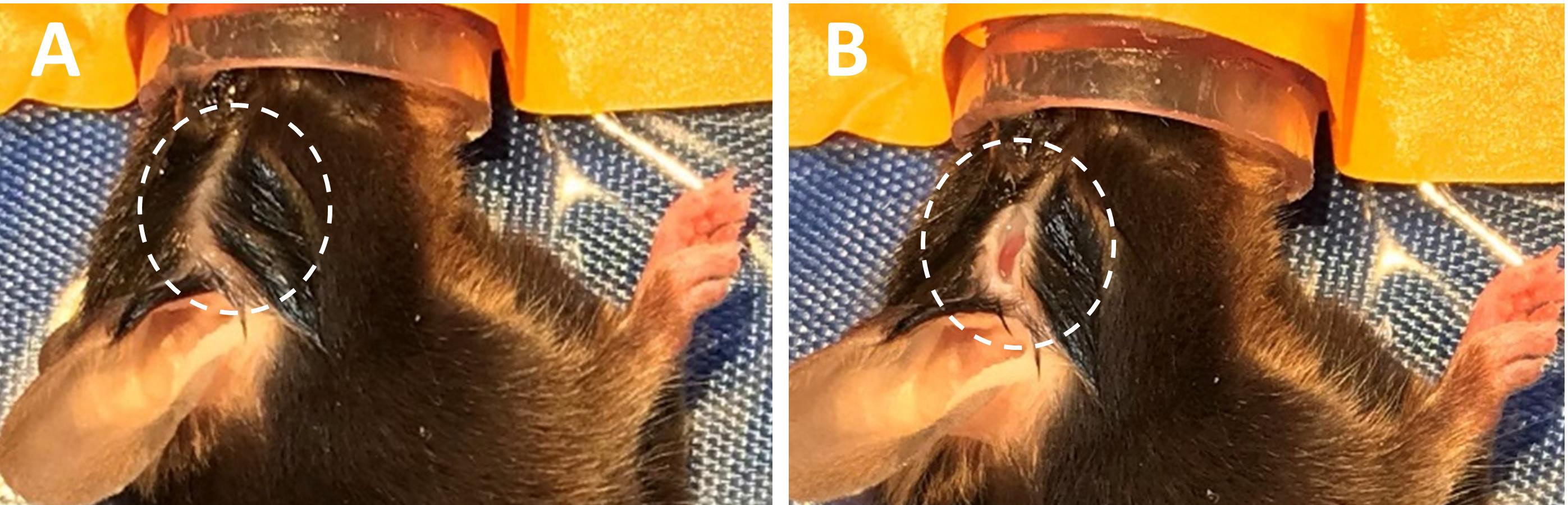
Figure 1. Make incision for LDF fiber. A. Prepare area between the right eye and right ear for incision. B. make a small incision in the prepped area. LDF, Laser Doppler Flowmetry.Place mouse in supine position and secure front legs to the warming pad with surgical tape.
Shave the ventral neck. Clean the area by alternating with chlorhexidine and 70% ethanol; repeat cleaning sequence for a total of three times.
Make a midline incision in the prepped ventral neck area. Blunt dissect to the left of the trachea (i.e., the animal’s right), moving the submandibular glands laterally. Continue the blunt dissection lateral to the sternohyoid and superiomedial to the sternocleidomastoid, until the carotid sheath is just visible (the vessel will be cleaned and exposed at a later step).
At the center of the midline incision, thread a suture (6-0 black braided suture with needle) through the right superficial infrahyoid muscles and the adjacent skin overlaying the trachea. Tie two double knots. Pull the loose end of the suture taut and to the right, to increase the surgical field of view. Secure the suture to the warming pad with surgical tape. Repeat this process with the skin and underlying tissue on the left side of the ventral neck incision (Figure 2).
Place the LDF optic fiber flat against the skull using the lateral incision made in Step B5 (Figure 2A-2B). Calibrate the LDF system and note the baseline value of cortical blood flow for the mouse.
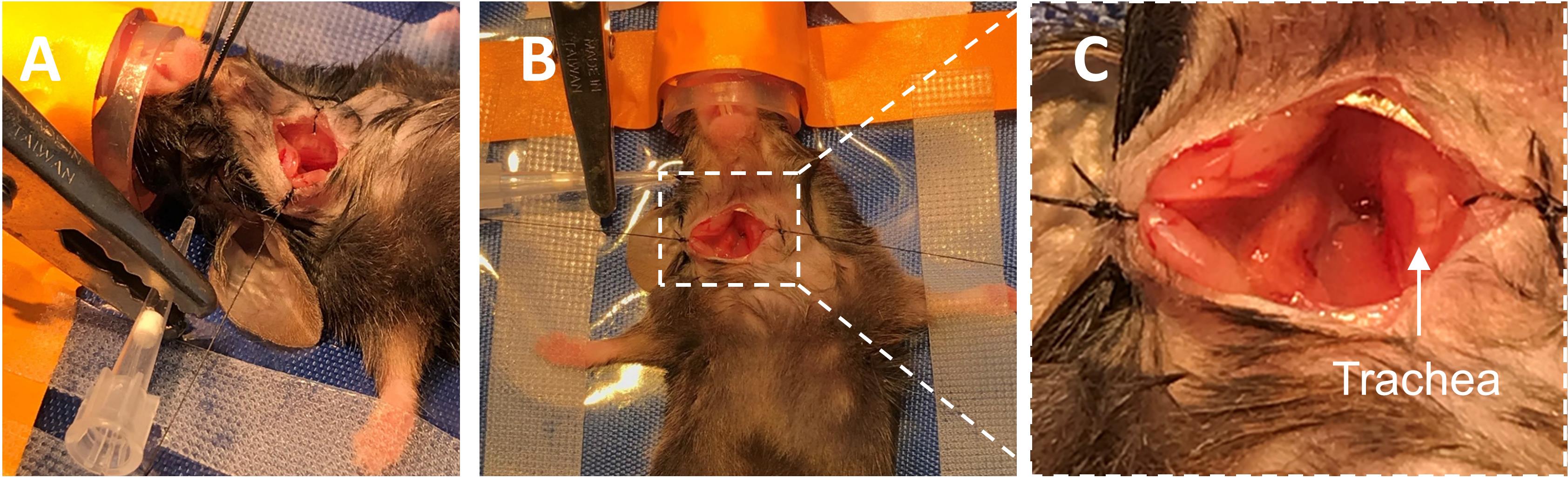
Figure 2. Make ventral neck incision and place LDF fiber against the skull. A. Lateral and B. ventral views of the neck incision pulled open with braided sutures, with the LDF fiber placed against the skull. C. Zoomed view of ventral neck incision pulled open. LDF, Laser Doppler Flowmetry.Locate the carotid sheath containing the CCA and vagus nerve (Figure 3A). Using blunt dissection, expose the CCA from within the sheath. Starting at the most proximal portion of the exposed CCA, blunt dissect up the vessel, coursing distally while cleaning structures from its walls. Take care not to injure the vagus nerve.
Upon reaching the carotid bifurcation, continue with blunt dissection of the ECA.
Branching laterally from the ECA is the occipital artery; leave this vessel intact.
Blunt dissect the connective tissue at the lateral edge of the hyoid bone, to better expose the ECA.
Branching medially from the ECA is the superior thyroid artery (STA); cauterize this vessel (Figure 3B-3C).
Note: This prevents the STA from rupturing when the monofilament is manipulated from the ECA to the ICA.
Blunt dissect the superior cervical ganglion from the CCA bifurcation, to fully expose the origin of the ICA (Figure 3D-3E).
Note: This is an optional step that can aid in visualization and make it easier to manipulate the monofilament from the ECA to ICA.
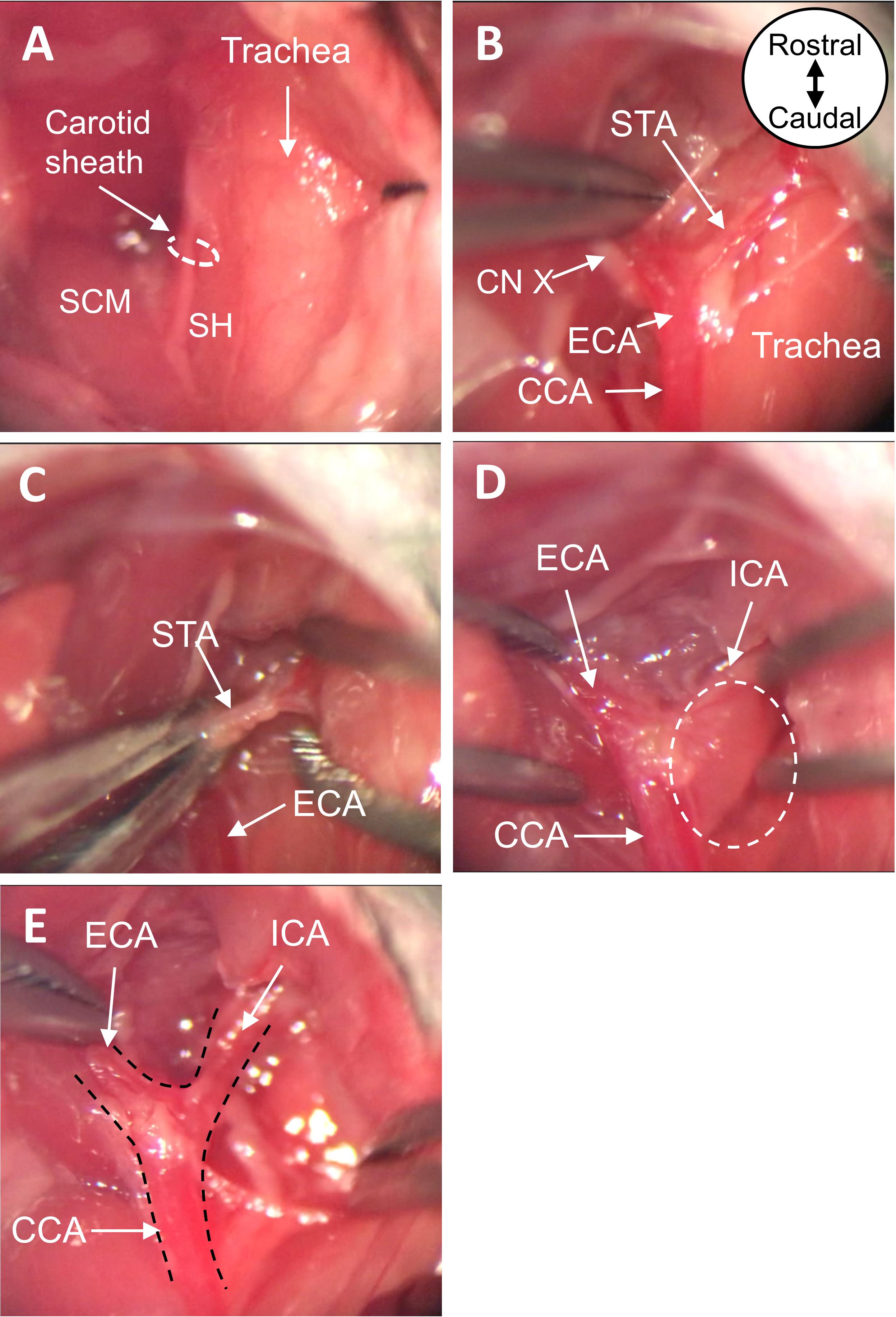
Figure 3. Expose CCA, ECA, and ICA. A. View of ventral neck incision before and B. after blunt dissection. C. Cauterize the STA with bipolar forceps. D. Detach the superior cervical ganglion at the carotid body from the carotid arteries. E. View of exposed CCA, ECA, and ICA after dissection. All images are ventral views oriented from rostral to caudal as depicted in panel B. CCA, common carotid artery; ECA, external carotid artery; ICA, internal carotid artery; STA, superior thyroid artery; SCM, sternocleidomastoid muscle; SH, sternohyoid muscle; CN X, vagus nerve.Slide two 6-0 black braided silk sutures underneath the ECA, such that the sutures are perpendicular to the vessel and parallel to one another. Grasping the more distal of the two sutures, create a loose knot around the ECA and gently slide it up the vessel, as far distal as possible. Tighten the suture knot to permanently ligate the ECA. Tape the ends of this suture to the nose cone, to gently pull the ECA taut (Figure 4A-4C).
Using the other 6-0 black braided silk suture, tie a loose knot around the ECA. Slide the suture knot distally up the ECA toward the permanent ligature made in Step B14, but do not tighten; this suture will be further manipulated in Step B17.
Place a curved micro clip across both the CCA and ICA, to block CCA blood flow from reaching the bifurcation and prevent backflow from the ICA (Figure 4D).
Note: To ensure proper occlusion, confirm the only structures in the micro clip are the CCA and ICA; adjust/replace micro clip as needed.
Move the loose suture knot (made in Step B15) proximally toward the origin of the ECA; do not tighten (Figure 4E).
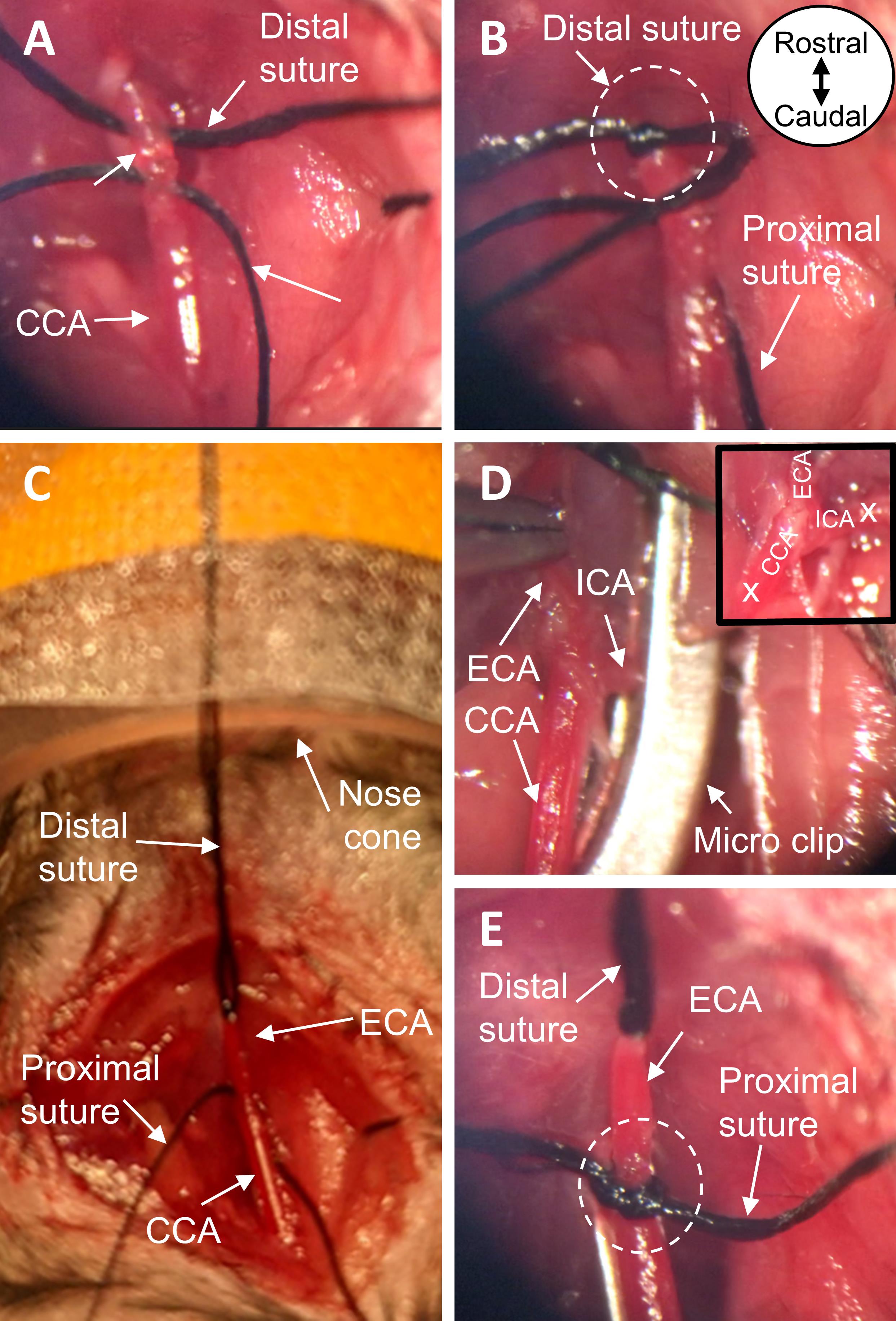
Figure 4. Prepare the carotid arteries for insertion of the monofilament. A. Place two black braided silk sutures under the ECA. B. With one suture, permanently ligate the most distal end of the exposed ECA. C. Tape the ends of the suture to the nose cone, pulling the ECA taut. D. Place a micro clip across both the CCA and ICA, to block blood flow at the carotid bifurcation. E. Using the remaining suture, tie a loose knot around the proximal end of the ECA near its origin. All images are ventral views oriented from rostral to caudal as depicted in panel B. ECA, external carotid artery; CCA, common carotid artery; ICA, internal carotid artery.Make an incision in the ECA between the two suture knots, approximately 2 mm proximal from the permanent ligation (distal suture knot) (Figure 5A). Insert the silicone rubber-coated tip of the monofilament into the ECA, directed toward the carotid bifurcation (Figure 5B). Position the loose suture knot such that it is around the portion of the ECA containing the coated tip; tighten the knot (Figure 5C).
Note: The suture knot needs to be tight enough so that the monofilament won’t be pushed out of the ECA when the vessel is reperfused, but also loose enough so that the monofilament can be progressed through the suture knot at a later step.
Remove the micro clip. Without cutting the monofilament, transect the ECA at the incision site, between the two suture knots, creating a proximal ECA stump containing the coated tip of the monofilament (Figure 5D-5E).
Advance the monofilament from the ECA into the ICA, via the CCA bifurcation. Once the coated tip of the monofilament progresses through the suture knot, tighten the knot to prevent blood loss. Continue to advance the monofilament into the ICA until the LDF shows a 40-70% decrease in cortical blood flow from baseline, indicating occlusion of the MCA. Once MCA occlusion is confirmed, secure the monofilament in place by further tightening the suture knot around the ECA stump (Figure 5F-5H).
Notes:
a. Confirm the monofilament has correctly advanced in the ICA and did not enter the pterygopalatine artery (PPA; branch of the ICA) (Figure 5G).
b. Occlusion of the MCA is achieved when the LDF indicates a 40-70% decrease in cortical blood flow from baseline, typically correlating with an insertion length of 6.5-10 mm (Figure 5H-5I).
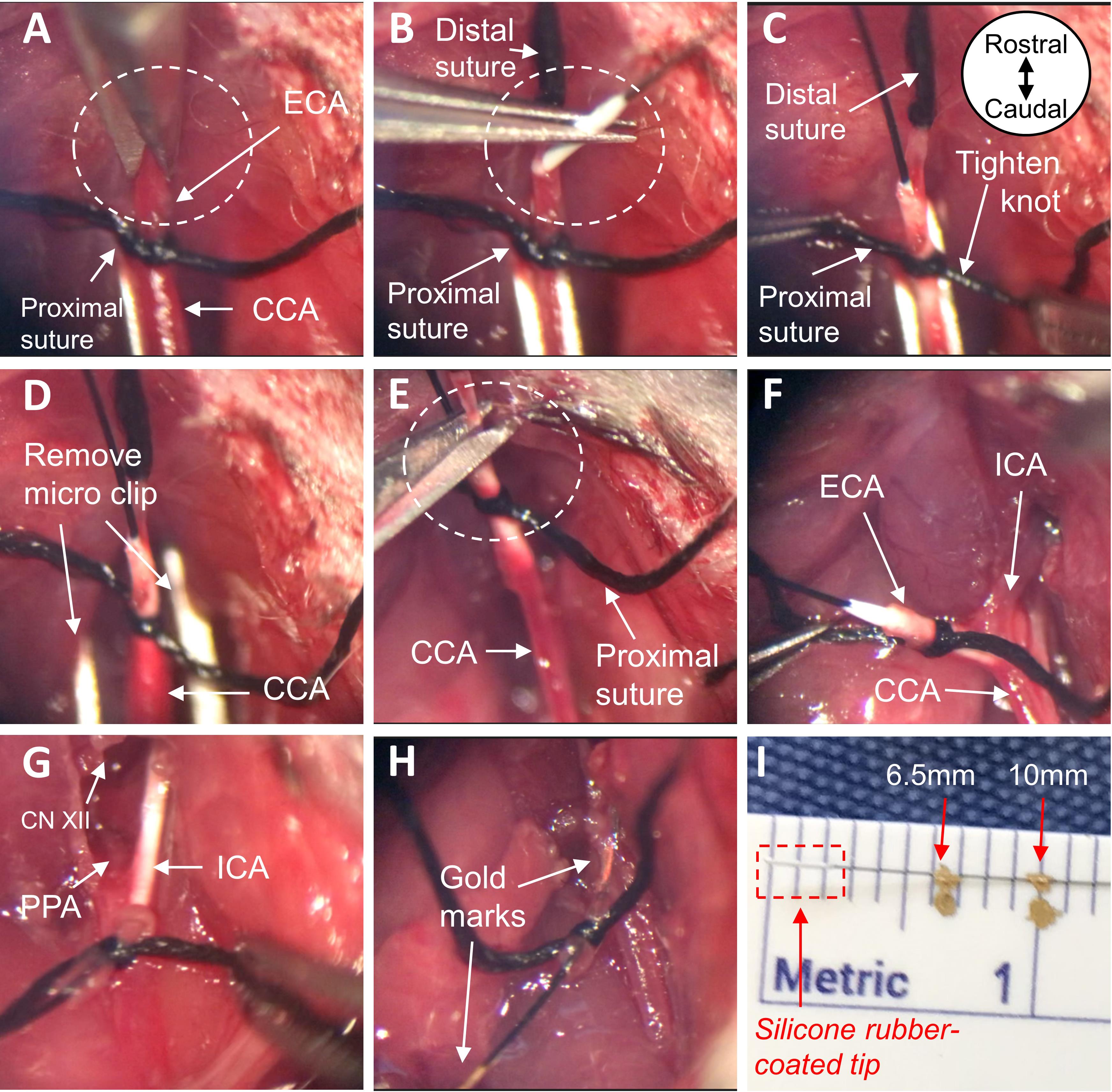
Figure 5. Insert the monofilament into the ICA. A. Make a small incision in the ECA and B. insert the coated tip of the monofilament. C. Tighten suture knot around ECA containing the coated tip. D. Remove the micro clip, restoring blood flow to the CCA and ICA, and E. transect the ECA at the monofilament entry site. F-G. Advance the monofilament by way of the CCA bifurcation into the ICA, carefully avoiding the PPA, until the monitored cortical blood flow decreases by 40-70% of baseline, H-I. typically correlating with an insertion length of 6.5-10 mm (distance delineated by gold marks in figure). All images are ventral views oriented from rostral to caudal as depicted in panel C. ECA, external carotid artery; CCA, common carotid artery; ICA, internal carotid artery; PPA, pterygopalatine artery; CN XII, hypoglossal nerve.Trim the end of the monofilament so that it can fit just under the skin, when the ventral neck incision is closed.
Note: Once the ventral neck incision is closed, the monofilament will be physically constrained by both the suture knot (made in Step B20) and the skin. If the suture knot loosens during the occlusion period, the additional physical constraint of the skin can help prevent the monofilament from moving out of the ICA/ECA.
Close the neck with AutoClips; close the scalp incision with tissue adhesive (Figure 6).
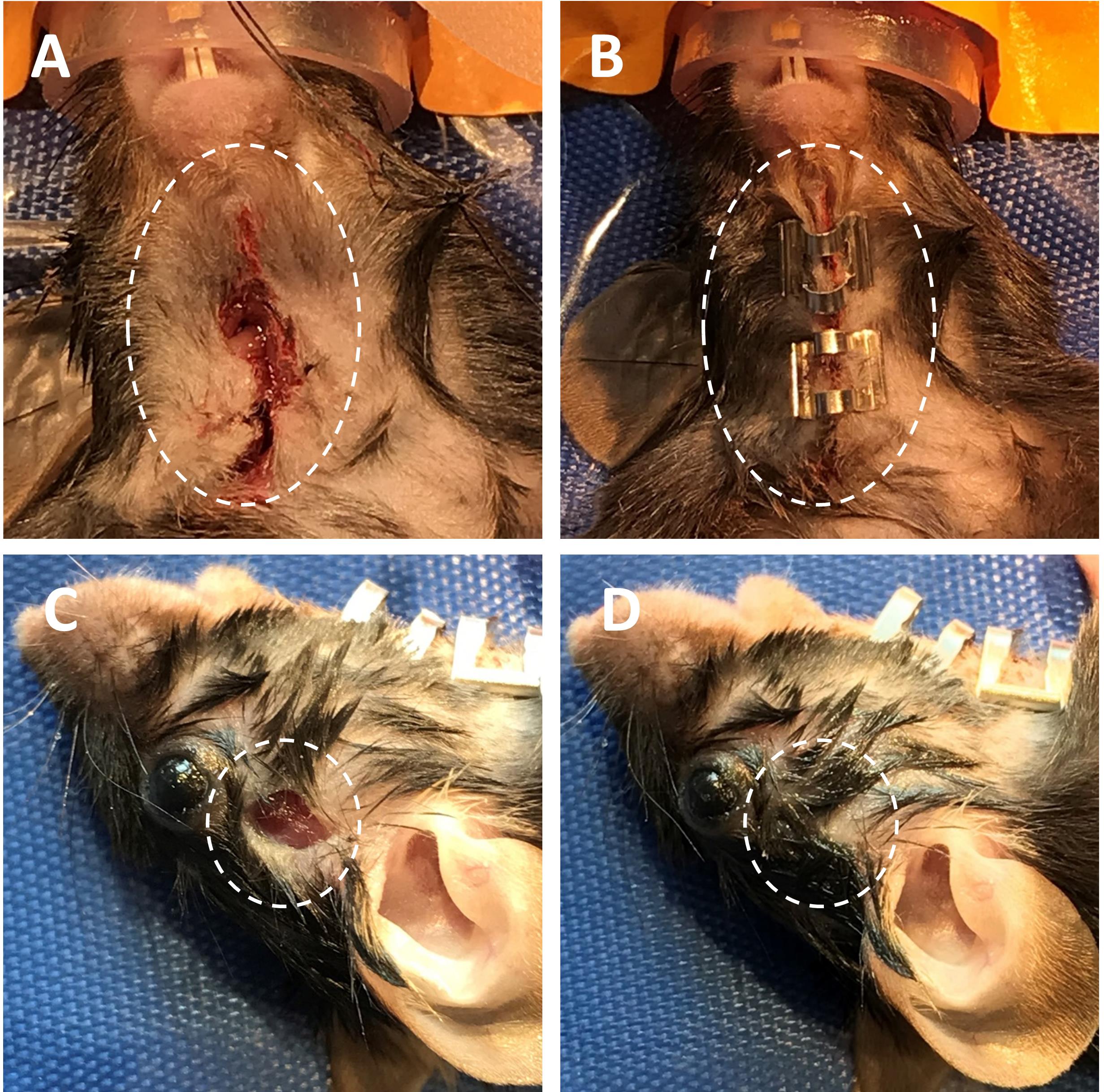
Figure 6. Close neck and scalp incisions. A-B. Temporarily close the ventral neck incision using AutoClips (ventral view). C-D. Close scalp incision with tissue adhesive (lateral view).Remove the mouse from the nose cone and place in new sterile cage during the ischemic event (either 30, 45, 60, or 90 min of occlusion time).
Upon completion of the occlusion period, re-anesthetize the mouse and place it in a supine position at the nose cone.
Remove AutoClips from the neck incision.
To allow reperfusion of the MCA-territory, begin by grasping one side of the proximal ECA knot (tightened in Step B20) and, using another pair of forceps in the other hand, grasp the monofilament. Slowly retract the monofilament out of the ECA stump, and quickly tighten the suture knot around the ECA stump to prevent blood loss (Figure 7). Trim excess suture length, leaving a few millimeters on both sides of the knot.
Notes:
a. Minor blood loss is to be expected upon removing the monofilament.
b. When the monofilament is removed, the silicone rubber coating will remain in the proximal ICA.
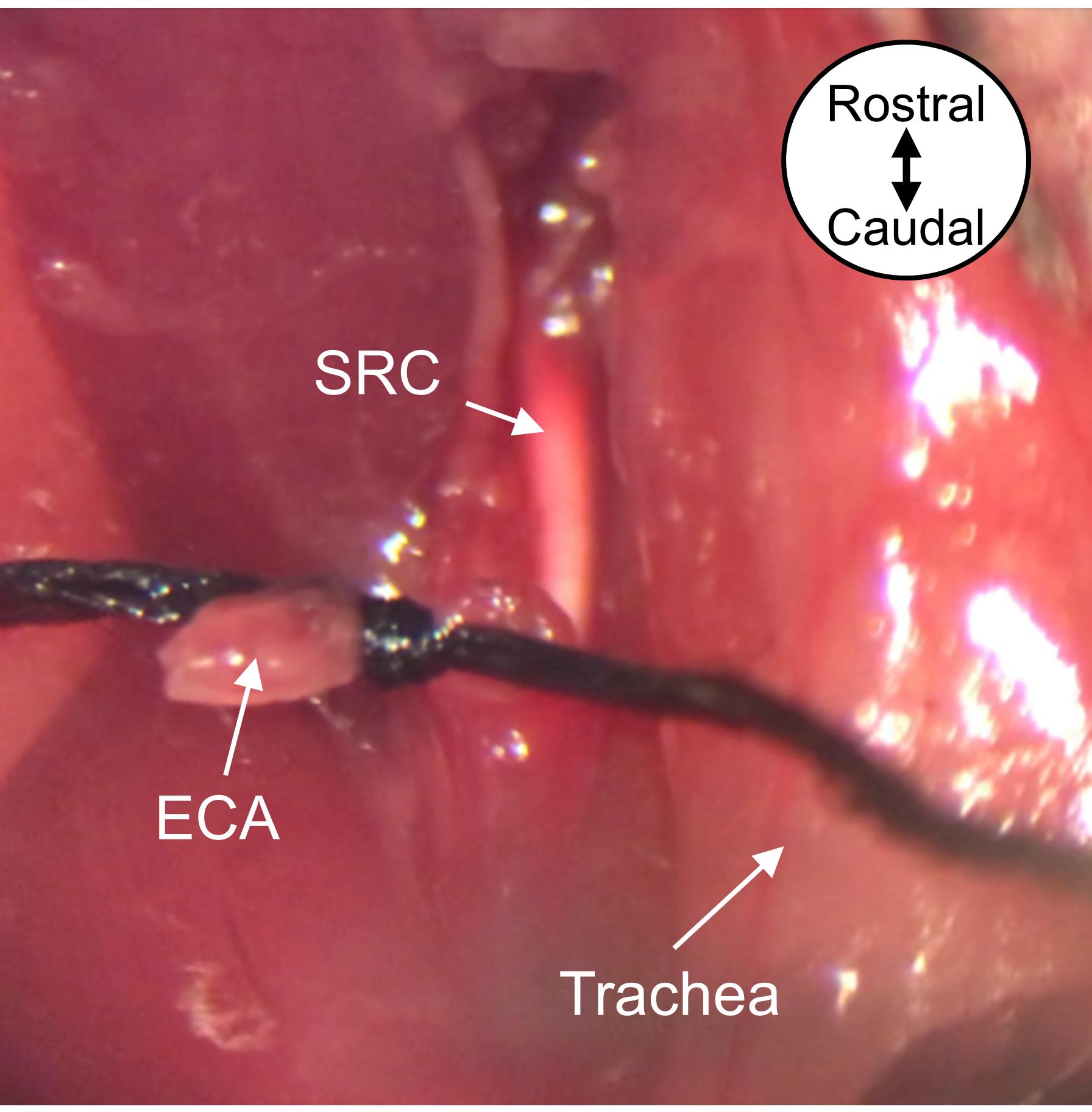
Figure 7. Remove the monofilament to allow reperfusion of MCA-supplied tissue. Remove the monofilament from the ECA and tighten the proximal ECA suture knot around the ECA stump. The silicone rubber coating from the monofilament tip will remain in the ICA. Image is a ventral view oriented from rostral to caudal as depicted. MCA, middle cerebral artery; ECA, external carotid artery; SRC, silicone rubber coating.Close the ventral neck incision with 6-0 braided absorbable sutures in a single interrupted suture pattern.
Subcutaneously inject 0.5-1.0 mL of warm saline into the mouse, to facilitate recovery and account for any blood loss during surgery.
Remove the mouse from the nose cone and place it back into the sterile cage. Allow the mouse to recover. Perform a visual neurological assessment.
Note: Typical neurological deficits include hemiparesis, weakness, and lethargy.
Post-operative Supportive Care
Closely monitor the mouse for the first 4 h after surgery.
Maintain mice on supplemented heat (e.g., using a slide warmer with temperature control) throughout the duration of the study.
Note: After MCAO, rodents cannot thermoregulate (Barber et al., 2004). Supplemented heat promotes survivability.
Provide mice with mash (i.e., wetted food) and hydrogel in Petri dishes on the cage floor, to encourage feeding/fluid intake.
Note: Provide mash and hydrogel for at least 7-14 days post-surgery, or until the mouse is able to eat sufficiently from the standard cage feeder. This helps address multiple considerations including blood loss, post-operative weight loss, and consequences of ECA ligation. Change mash and hydrogel daily. If the mouse continues to have difficulty rearing/eating normally, provide mash throughout the duration of the study.
Administer warm saline (subcutaneous injection) daily for the first 7 days. Monitor weight daily. If fluid intake is insufficient, or if dehydration is suspected, warm saline injections should continue past the first 7 days.
Note: Take care to ensure saline is warm, not hot or cold, prior to injection. Warm saline helps reduce discomfort, factors in thermoregulatory issues, counters blood loss, and prevents dehydration. Dehydration can be assessed by performing a skin turgor test.
Continue to closely monitor the mice daily following surgery, for the presence of surgical complications or signs of pain or distress (e.g., no grooming, penile prolapse, penile plugs) until euthanasia.
Data analysis
Brain Magnetic Resonance Imaging (MRI) is often used to verify MCA-territory stroke and calculate infarct volume. Following T2-weighted imaging, digital planimetry is performed to quantify infarct area on each coronal slice. Infarct areas are then summed, multiplied by slice thickness, and corrected for edema, to determine infarct volume as previously described (Lemmerman et al., 2021). Other approaches include 2,3,5-Triphenyltetrazolium chloride (TTC) staining of the brain at harvest, or various histochemical/histological analyses (e.g., H&E, Nissl).
When utilizing the intraluminal tMCAO model, sample sizes were estimated based on a power analysis and our prior work in this model. Of 334 tMCAO procedures performed by our dedicated small animal surgeon over the course of one year, less than 5% of animals died during surgery or as a result of surgical complications. However, to account for exclusion criteria and mortality associated with the procedure, we suggest researchers increase their sample size estimates by 20%.
Notes
The following information details additional recommendations for the surgical procedure. First, we suggest using an anesthesia nose cone with a circular opening shape (we created this nose cone in house), rather than asymmetrical; this allows better anesthesia flow and lets the mouse be shifted easily from a side position to supine, without needing to adjust the position of the nose cone. To secure the LDF optic fiber in place, we recommend placing the fiber through a filtered pipette tip. Make a small hole in the filter, such that the fiber can fit tightly. Progress the fiber through the new hole until the end of the fiber is extended a few millimeters past the narrow end of the pipette tip. The pipette tip, now containing the optic fiber, can then be clamped by an adjustable mechanical arm, permitting the optic fiber to be set in position against the skull and held consistently throughout the length of the surgery. To ensure preparation in the instance that a vessel would rupture during surgery, we suggest having a straight micro clip readily available to quickly clamp the vessel. If a vessel ruptures, apply pressure with a Q-tip and use the straight micro clip to temporarily clamp the vessel. Clean the area of excess blood and locate the site of rupture. If a small branch is the source, cauterization may be useful. However, if the CCA, PPA, or ICA are the source, euthanasia is warranted. Notably, vessels are more prone to rupturing when the tissue is dry; therefore, we also recommend having saline readily available to wet the exposed tissue, if observed to be drying out. Please see Supplementary Materials for additional images of supplies, reagents, and surgical setup.
For the purpose of estimating the duration of the procedure and recovery period, we suggest anticipating 60-90 minutes from initial induction with isoflurane (Procedure Step B1) to completion of the procedure (Procedure Step B29). Note that this estimate does not include the duration of the occlusion period (Procedure Step B23). Once the procedure is complete and the animal is in its cage for post-operative recovery, the animal should be awake and alert within 5-10 minutes of removal from anesthesia, though activity will be low.
Variations in outcomes such as infarct size and mortality can be attributed to (a) experimental/procedural design, (b) animal physiology, or (c) performance of the surgeon.
(a) Experimental design factors, such as monofilament tip size, duration of occlusion, LDF readings, and mouse characteristics can affect stroke severity. The size of the monofilament tip must be chosen carefully; if too large, it may not advance far enough, thus occluding the ICA; if too small, the tip may advance past the marked insertion length without MCA occlusion, and the lack of resistance may prompt accidental puncturing of the vessel, causing a hemorrhage and/or death. The duration of occlusion should be carefully chosen, based on the current literature and intentions of the study, as longer occlusions will produce larger infarcts. LDF is used to confirm reduction of blood flow into the MCA and must be monitored carefully to verify the necessary 40-70% drop in blood flow. It is important to note that, if the LDF system is not working correctly, this can affect monofilament tip placement and thus alter infarct volume. Mouse characteristics such as age, sex, and housing status (single versus group) should also be considered in light of anticipated outcomes. Our group most commonly performs this procedure on mice aged 10-12 weeks (Lemmerman et al., 2021). Finally, selection of murine models must factor in the known differences in vessel collateralization, infarct volume, and stroke variability between strains (Fluri et al., 2015).
(b) Some variation presents because of factors inherent to the animal. Aside from the aforementioned consideration of mouse strain, animals with anatomical variations in vasculature (e.g., abnormal arterial branching pattern), unknown comorbidities (e.g., cancer), or unforeseen intolerance of anesthesia may experience higher mortality or variation in stroke severity and ischemic territory. As such, detailed note-taking of observations before MCAO, during surgery, and post-operatively through the duration of the study is imperative.
(c) The skillset of the surgeon is a major contributor to the success of the procedure and outcomes. Maintaining suitable anesthetic plane, ensuring proper insertion length of the monofilament, keeping duration of surgery to an appropriate minimum for the health of the animal, as well as being prepared with the necessary anatomical knowledge and fine technical skills are vital. In our group, we have a dedicated small animal surgeon on staff with eight years of surgical experience. Our surgeon began by observing the procedure routinely for approximately one month (please see Video 1, if observation is not possible), followed by over three months of practice, during which approximately two surgeries with a 60 min occlusion were performed daily. Post-operative care was practiced during this time. The goal was to have animals survive to 48 hours and, after euthanasia, confirm successful MCA-territory stroke with TTC staining. Because skillset and technical ability may vary significantly from person to person, the number of mice required to achieve consistent data will equally vary.
If interested in neurogenesis, neuroprotection, or neuroinflammation, the use of analgesics should be carefully reviewed. Studies showed that certain anti-inflammatory drugs reduce infarct volume after stroke, and use of more potent pain medication diminished alertness, activity, and eating/drinking behavior (Jacobsen et al., 2013; Pettit et al., 2012). Finally, though animals can remain under anesthesia during the occlusion period, isoflurane anesthesia is reported to be somewhat neuroprotective (Bickler and Fahlman, 2006) and vasodilative (Iida et al., 1998). As such, means of minimizing animal stress, while emulating the consciousness and cerebrovascular state of human stroke, must be addressed appropriately (Sicard et al., 2003).
Appropriate controls for this MCAO mouse model include an experimental group of naïve mice (i.e., healthy no surgery) and/or an experimental group of mice subjected to a sham MCAO procedure (i.e., without placement of silicone rubber coated monofilament). To perform a sham MCAO procedure, complete all protocol steps as written through Step B14. Next, using the other 6-0 black braided silk suture, tie a loose knot around the ECA. Move the loose suture knot proximally towards the origin of the ECA and tighten. Then, transect the ECA between the two suture knots and trim excess suture length. Skipping ahead, perform Step B27 and close the scalp incision with tissue adhesive. Finally, perform Steps B28-C5.
Recipes
70% Ethanol solution
Mix 7 mL of 100% Ethanol with 3 mL of sterile water, to create 10 mL of 70% ethanol solution.
Acknowledgments
The graphical abstract was created with BioRender.com. Funding for this work was partly provided by New Innovator Award DP2EB028110 (NIBIB/NIH), and DP1DK126199 (NIDDK/NIH) to DGP. This protocol was used in mice, or modified for use in rats, in the following research studies (Khanna et al., 2017; Rink et al., 2017; Sen et al., 2017; Balch et al., 2021; Lemmerman et al., 2021 [DOI: 10.1126/sciadv.abd4735]).
Competing interests
No potential competing interest was reported by the authors.
Ethics
All animal experiments were performed in accordance with protocols approved by the Laboratory Animal Care and Use Committee at The Ohio State University (IACUC # 2016A00000074-R1 and IACUC # 2009A0006-R3).
References
- Adams, H. P., Jr. and Nudo, R. J. (2013). Management of patients with stroke: is it time to expand treatment options? Ann Neurol 74(1): 4-10.
- Albers, G. W., Marks, M. P., Kemp, S., Christensen, S., Tsai, J. P., Ortega-Gutierrez, S., McTaggart, R. A., Torbey, M. T., Kim-Tenser, M., Leslie-Mazwi, T., et al. (2018). Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med 378(8): 708-718.
- Balch, M. H. H., Harris, H., Chugh, D., Gnyawali, S., Rink, C., Nimjee, S. M. and Arnold, W. D. (2021). Ischemic stroke-induced polyaxonal innervation at the neuromuscular junction is attenuated by robot-assisted mechanical therapy. Exp Neurol 343: 113767.
- Barber, P. A., Hoyte, L., Colbourne, F. and Buchan, A. M. (2004). Temperature-regulated model of focal ischemia in the mouse: a study with histopathological and behavioral outcomes. Stroke 35(7): 1720-1725.
- Benjamin, E. J., Muntner, P., Alonso, A., Bittencourt, M. S., Callaway, C. W., Carson, A. P., Chamberlain, A. M., Chang, A. R., Cheng, S., Das, S. R., et al. (2019). Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 139(10): e56-e528.
- Bickler, P. E. and Fahlman, C. S. (2006). The inhaled anesthetic, isoflurane, enhances Ca2+-dependent survival signaling in cortical neurons and modulates MAP kinases, apoptosis proteins and transcription factors during hypoxia. Anesth Analg 103(2): 419-429, table of contents.
- Carmichael, S. T. (2005). Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx 2(3): 396-409.
- Chan, P. H., Kamii, H., Yang, G., Gafni, J., Epstein, C. J., Carlson, E. and Reola, L. (1993). Brain infarction is not reduced in SOD-1 transgenic mice after a permanent focal cerebral ischemia. Neuroreport 5(3): 293-296.
- Diseases, G. B. D. and Injuries, C. (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396(10258): 1204-1222.
- Dittmar, M., Spruss, T., Schuierer, G. and Horn, M. (2003). External carotid artery territory ischemia impairs outcome in the endovascular filament model of middle cerebral artery occlusion in rats. Stroke 34(9): 2252-2257.
- Engel, O., Kolodziej, S., Dirnagl, U. and Prinz, V. (2011). Modeling stroke in mice - middle cerebral artery occlusion with the filament model. J Vis Exp (47). 2423.
- Evans, M. R. B., White, P., Cowley, P. and Werring, D. J. (2017). Revolution in acute ischaemic stroke care: a practical guide to mechanical thrombectomy. Pract Neurol 17(4): 252-265.
- Fluri, F., Schuhmann, M. K. and Kleinschnitz, C. (2015). Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther 9: 3445-3454.
- Hacke, W., Kaste, M., Bluhmki, E., Brozman, M., Davalos, A., Guidetti, D., Larrue, V., Lees, K. R., Medeghri, Z., Machnig, T., et al. (2008). Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359(13): 1317-1329.
- Hermann, D. M., Popa-Wagner, A., Kleinschnitz, C. and Doeppner, T. R. (2019). Animal models of ischemic stroke and their impact on drug discovery. Expert Opin Drug Discov 14(3): 315-326.
- Iida, H., Ohata, H., Iida, M., Watanabe, Y. and Dohi, S. (1998). Isoflurane and sevoflurane induce vasodilation of cerebral vessels via ATP-sensitive K+ channel activation. Anesthesiology 89(4): 954-960.
- Jacobsen, K. R., Fauerby, N., Raida, Z., Kalliokoski, O., Hau, J., Johansen, F. F. and Abelson, K. S. (2013). Effects of buprenorphine and meloxicam analgesia on induced cerebral ischemia in C57BL/6 male mice. Comp Med 63(2): 105-113.
- Kaiser, E. E. and West, F. D. (2020). Large animal ischemic stroke models: replicating human stroke pathophysiology. Neural Regen Res 15(8): 1377-1387.
- Khanna, S., Stewart, R., Gnyawali, S., Harris, H., Balch, M., Spieldenner, J., Sen, C. K. and Rink, C. (2017). Phytoestrogen isoflavone intervention to engage the neuroprotective effect of glutamate oxaloacetate transaminase against stroke. FASEB J 31(10): 4533-4544.
- Kumar, A., Aakriti and Gupta, V. (2016). A review on animal models of stroke: An update. Brain Res Bull 122: 35-44.
- Lee, S., Lee, M., Hong, Y., Won, J., Lee, Y., Kang, S. G., Chang, K. T. and Hong, Y. (2014). Middle cerebral artery occlusion methods in rat versus mouse models of transient focal cerebral ischemic stroke. Neural Regen Res 9(7): 757-758.
- Lemmerman, L. R., Balch, M. H. H., Moore, J. T., Alzate-Correa, D., Rincon-Benavides, M. A., Salazar-Puerta, A., Gnyawali, S., Harris, H. N., Lawrence, W., Ortega-Pineda, L., et al. (2021). Nanotransfection-based vasculogenic cell reprogramming drives functional recovery in a mouse model of ischemic stroke. Sci Adv 7(12): eabd4735.
- Mokin, M., Pendurthi, A., Ljubimov, V., Burgin, W. S., Siddiqui, A. H., Levy, E. I. and Primiani, C. T. (2018). ASPECTS, Large Vessel Occlusion, and Time of Symptom Onset: Estimation of Eligibility for Endovascular Therapy. Neurosurgery 83(1): 122-127.
- Narayan, S. K., Grace Cherian, S., Babu Phaniti, P., Babu Chidambaram, S., Rachel Vasanthi, A. H. and Arumugam, M. (2021). Preclinical animal studies in ischemic stroke: Challenges and some solutions. Animal Model Exp Med 4(2): 104-115.
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. (1995). Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333(24): 1581-1587.
- Nogles, T. E. and Galuska, M. A. (2021). Middle Cerebral Artery Stroke. StatPearls.
- Nogueira, R. G., Jadhav, A. P., Haussen, D. C., Bonafe, A., Budzik, R. F., Bhuva, P., Yavagal, D. R., Ribo, M., Cognard, C., Hanel, R. A., et al. (2018). Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med 378(1): 11-21.
- Pettit, A. S., Desroches, R. and Bennett, S. A. (2012). The opiate analgesic buprenorphine decreases proliferation of adult hippocampal neuroblasts and increases survival of their progeny. Neuroscience 200: 211-222.
- Pierot, L., Jayaraman, M. V., Szikora, I., Hirsch, J. A., Baxter, B., Miyachi, S., Mahadevan, J., Chong, W., Mitchell, P. J., Coulthard, A., et al. (2018). Standards of practice in acute ischemic stroke intervention: international recommendations. J Neurointerv Surg 10(11): 1121-1126.
- Powers, W. J., Rabinstein, A. A., Ackerson, T., Adeoye, O. M., Bambakidis, N. C., Becker, K., Biller, J., Brown, M., Demaerschalk, B. M., Hoh, B., et al. (2018). 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 49(3): e46-e110.
- Rink, C., Gnyawali, S., Stewart, R., Teplitsky, S., Harris, H., Roy, S., Sen, C. K. and Khanna, S. (2017). Glutamate oxaloacetate transaminase enables anaplerotic refilling of TCA cycle intermediates in stroke-affected brain. FASEB J 31(4): 1709-1718.
- Saver, J. L., Albers, G. W., Dunn, B., Johnston, K. C., Fisher, M. and Consortium, S. V. (2009). Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke 40(7): 2594-2600.
- Sen, C. K., Khanna, S., Harris, H., Stewart, R., Balch, M., Heigel, M., Teplitsky, S., Gnyawali, S. and Rink, C. (2017). Robot-assisted mechanical therapy attenuates stroke-induced limb skeletal muscle injury. FASEB J 31(3): 927-936.
- Sicard, K., Shen, Q., Brevard, M. E., Sullivan, R., Ferris, C. F., King, J. A. and Duong, T. Q. (2003). Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J Cereb Blood Flow Metab 23(4): 472-481.
- Smith, W. S. and Furlan, A. J. (2016). Brief History of Endovascular Acute Ischemic Stroke Treatment. Stroke 47(2): e23-26.
- Virani, S. S., Alonso, A., Aparicio, H. J., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., Carson, A. P., Chamberlain, A. M., Cheng, S., Delling, F. N., et al. (2021). Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 143(8): e254-e743.
- Yaghi, S., Willey, J. Z., Cucchiara, B., Goldstein, J. N., Gonzales, N. R., Khatri, P., Kim, L. J., Mayer, S. A., Sheth, K. N., Schwamm, L. H., et al. (2017). Treatment and Outcome of Hemorrhagic Transformation After Intravenous Alteplase in Acute Ischemic Stroke: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 48(12): e343-e361.
Supplementary information
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Lemmerman, L. R., Harris, H. N., Balch, M. H. H., Rincon-Benavides, M. A., Higuita-Castro, N., Arnold, W. D. and Gallego-Perez, D. (2022). Transient Middle Cerebral Artery Occlusion with an Intraluminal Suture Enables Reproducible Induction of Ischemic Stroke in Mice. Bio-protocol 12(3): e4305. DOI: 10.21769/BioProtoc.4305.
- Lemmerman, L. R., Balch, M. H. H., Moore, J. T., Alzate-Correa, D., Rincon-Benavides, M. A., Salazar-Puerta, A., Gnyawali, S., Harris, H. N., Lawrence, W., Ortega-Pineda, L., et al. (2021). Nanotransfection-based vasculogenic cell reprogramming drives functional recovery in a mouse model of ischemic stroke. Sci Adv 7(12): eabd4735.
Category
Neuroscience > Nervous system disorders > Stroke
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










