- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A low-cost Portable Device to Deliver Smoke, Volatile or Vaporized Substances to Drosophila melanogaster , Useful for Research and/or Educational Assays
Published: Vol 11, Iss 23, Dec 5, 2021 DOI: 10.21769/BioProtoc.4244 Views: 3103
Reviewed by: Norma Velazquez UlloaIvan Sanchez DiazAnonymous reviewer(s)
Abstract
Drosophila melanogaster has been used to test drugs of abuse, substances with potential benefits for medical purposes, as well as contaminants and hazardous volatile compounds. This model has also been used for the characterization of behavioral changes, physiopathological consequences, and subcellular mechanisms of the use of cocaine, methamphetamines, ethanol, nicotine, cannabinoids, toluene, and other airborne volatile organic compounds. When testing these substances, routes of administration are important to define. Admixing the test compounds with water or food is one suitable option in many cases, but the inhalation route is especially suitable when the administration of one or more volatile compounds is desired. One advantage of the administration of substances via the inhalation route is its rapid exchange and distribution throughout the cuticle and the tracheal system. In addition, this route allows treating a large group of individuals simultaneously. Moreover, the inhalation route is frequently used to administer different drugs to humans. A good model system shares physiology and molecular pathways with humans, and D. melanogaster possesses almost 75% homologous genes associated with human diseases. Methodologies to deliver the abovementioned substances usually include customized devices. Herein, we focus on the development of a low-cost customized device useful to deliver smoke or vaporizable compounds to D. melanogaster. This approach might be applied for acute or chronic exposure to vaporized substances. In particular, our device was utilized for testing cigarette smoke and vaporized cannabis extract on cardiac performance of adult individuals during chronic treatment. We are describing how to set up this low-cost portable device, useful for research and/or educational assays, taking advantage of the amenability of D. melanogaster to test different compounds in relatively short periods, and especially including a large number of individuals at the same time.
Graphic abstract:

Custom-made device useful for inhalation pathway assays in Drosophila melanogaster.
Background
Drosophila melanogaster is a useful model for studying drugs of abuse, namely nicotine, cocaine, alcohol, and methamphetamines (Bainton et al., 2000; Velazquez-Ulloa, 2017; Morris et al., 2018; Chvilicek et al., 2020; Baker et al., 2021; Park et al., 2021), as well as substances with therapeutic potential as cannabis (Gómez et al., 2019). Moreover, this model organism has been utilized for studies of toxins (Broderick et al., 2006) and atmospheric pollutants (Wilson et al., 2005). This analysis involves physiological responses (Wilson et al., 2005; Gómez et al., 2019; Santalla et al., 2021), modulation of behavior (Bainton et al., 2000; Rigo et al., 2021), subcellular mechanisms (Petruccelli et al., 2018; Shohat-Ophir et al., 2012), and the relevance of genetic mutations (Sanchez-Díaz et al., 2015; Petruccelli et al., 2018; Rigo et al., 2021). Substances have been administrated orally as supplemented food, including through methods as the CApillary FEeder (CAFE) Assay (Sanchez-Díaz et al., 2015; Baker et al., 2021), or volatilized by use of specific devices (McClung and Hirsh, 1998; Filošević et al., 2018).
One of the main reasons for choosing the passive inhalation route relates to the drawback of oral administration of substances with bad taste, as a fly is not prone to eat drugs with bad taste (Pandey and Nichols, 2011). Advantageously, the inhalation pathway allows the delivery of drugs in a short period of time. The delivery of substances via inhalation increases the availability of the drug and the rate of absorption throughout the cuticle, evading the digestive tract. This way of administration also allows treating a large number of individuals simultaneously, instead of using injection procedures that require one-by-one application. Taking advantage of the demonstrated usefulness of D. melanogaster in modeling various human diseases, this route of administration is suitable for administrating drugs that humans typically consume by the inhalation route, like cannabis. Additionally, D. melanogaster is usually used to test air contaminants by means of this administration route. Different devices designed to provide substances to D. melanogaster include commercial components, are usually expensive, or have a complex design that hampers easy disassembly, transportation, and reassembly (Linde et al., 2014; Tatum-Gibbs et al., 2015).
In a recent publication, we assessed the effect of nicotine on the cardiac performance of Drosophila melanogaster. We found that chronic treatment of adult wild-type flies with vaporized nicotine increased heart rate and altered cardiac contraction/relaxation dynamics (Santalla et al., 2021). Experiments from our laboratory have shown that vaporized cannabis extract also modified heart activity. We observed that a short treatment with cannabis (vaporization of cannabis extract) increased the propensity to cardiac arrhythmias, while a prolonged cannabis dosage enhanced cardiac contractility (Gómez et al., 2019). Herein, we describe the method used for administrating smoke or vaporized substances to Drosophila melanogaster using an easy to mount and low-cost custom device. The protocol allows to simultaneously expose several flies to the substance, thereby saving time and reagents. Although our previously published articles describe the effects of tobacco smoke and vaporized cannabis on D. melanogaster cardiac performance, herein, we are emphasizing the assembly of the device, its maintenance, and the procedure for administrating substances. The experimental amenability of the fruit fly model provides key insights into physiopathological responses to test substances and the subcellular mechanisms involved, many of them conserved between taxa. Noteworthy, the fruit fly is an excellent model for educational purposes. By combining the feasibility of Drosophila and the portability of our small device, simple assays might be conducted for research and education purposes.
Materials and Reagents
Polystyrene fly vials and plugs (outer diameter: 25 mm, height: 95 mm. Flystuff, Superbulk® Pack Vials, catalog number: 32-113RL)
Regular cotton balls (e.g., Amazon.com Inc., Kendall/Covidien Prepping Cotton Ball, 500 Count ID product: MD44KDL2600Z.BG or any cosmetic, perfumery, pharmaceutics store) or Drosophila plugs for vials (BIOLOGICS, catalog number: 51-0505)
Paint brushes for pushing flies (Round Pointed Tip Nylon Hair, size 4, any brand, e.g., Amazon Basics, ID product PBR-01)
Paper towels (e.g., Amazon.com, Inc., Scott Essential Multifold Paper Towels ID product: 01840)
Disposable cigarettes (any commercially available, e.g., Philip Morris box 20, ID product: 48). However, reusable e-cigarettes can be used (e.g., Epuffer, Snaps rev4 rechargeable e-cigarette, product ID: 299246).
Filter (optional, e.g., Amazon.com Inc, TarBust Disposable Cigarette Filters Bulk Economy Pack, 300 Per Pack, catalog number: M1200805)
Drosophila Canton S wild type flies (Bloomington Drosophila Resource Center # 64349)
Flies can be kept at room temperature (RT). Under our standard conditions, stocks are maintained in an incubator at 25°C and 60% relative humidity, in a 12/12 h light-dark cycle. However, these incubation conditions can be modified as desired by the users according to the purpose of their experiments.
Agarose (Sigma, catalog number: A9539)
Dextrose (Sigma, catalog number: G8270-1KG)
Yeast (Genesee Scientific, catalog number: 62-108)
Gelidium Agar (Genesee Scientific, catalog number: 66-105)
Cornmeal (Genesee Scientific, catalog number: 62-100)
10% Nipagin (Nipagin-Genesee Scientific, catalog number: 20-258)
96% ethanol (Sigma, catalog number: 793213)
Herb weed cannabis (PlantAR Ciencia, Registration number: 46875)
Custom fly food, easy to be prepared for flies’ stocks maintenance (see Recipes)
Cannabis treatment (see Recipes)
Equipment
Drosophila incubator (Sheldon Manufacturing, Shel Lab, catalog number: SRI20PF)
Stereo microscope (Carl Zeiss, model: Stemi 508, or any standard stereoscope)
CO2 fly pad (Genesee Scientific, catalog number: 59-114)
CO2 tank and regulator available in your location (e.g., AIRGAS.INC, Industrial Grade Carbon Dioxide, Size 150 High Pressure Aluminum Cylinder, CGA-320 ID product CD 35A)
Vaporizer (Davinci®, model: Iq2)
Any other model or brand might be useful. We used a vaporizer available for cannabis. The alternative for cannabis and extract might be useful in testing other substances. An important feature that the vaporizer must fulfill is to have a temperature regulator. This will allow vaporizing specific components at a preset temperature.
Multi-way stopcock manifold
Kits include several manifolds, valves, and fittings (e.g., PRIMER BIOSCIENCE, Luer valve assortment kit, catalog number: 14011). Otherwise, one manifold is enough (e.g., Scientific Commodities. Inc, catalog number: 8596). The unit should include at least 4 ports and two valves (see Figure 1)
Luer locks for manifold ports (PRIMER BIOSCIENCE, Luer valve assortment kit, catalog number: 14011; or they can be purchased separately, e.g., Coler Parmer, Masterflex® Adapter Fittings Luer to Plug, catalog number: EW-30800-30)
Translucent silicone tube (Scientific Commodities. Inc, catalog number: BB518-250: 6.35 mm × 9.53 mm, ID and OD respectively)
Translucent PVC tube (Scientific Commodities. Inc, catalog number: BB31775-V/18: 6.40 mm × 9.51 mm, ID and OD respectively)
Syringe (20 ml) (LABBOX LABWARE, catalog number: SYRL-020-080, or similar supplier)
Aspirator (PRIORI, ID product: PP1532)
Container box (any standard small carton box)
Tips for 100 µl automatic pipettes. They are adequate for ensuring a proper flux of vapor and smoke, avoiding flies to escape across the cotton ball or the plug (Sigma-Aldrich, Greiner pipette tips, catalog number: Z617369-15000EA or another provider).
Items 6 to 13 are shown in Figure 1.

Figure 1. Components required to assemble the device used in the protocol.
Procedure
Delivery of vaporized substances
Assembly and set up of the device (Figure 2)
Attach the vaporizer (Figure 2-I) to the device by connecting the mouthpiece of the vaporizer to the end of the manifold (Figure 2-II). A short silicone tube (Figure 2-III) might be used to adapt the mouthpiece to one end of the manifold.
Connect the syringe (Figure 2-IV) to the adjacent stopcock valve.
Connect one end of the PVC tubing (Figure 2-V) to the third stopcock valve. The other end must remain free.
Attach a 100 µl tip (Figure 2-VI) to the free end of the PVC tubing.
In case the manifold contains extra valves (i.e., three or four way stopcock manifold), they must remain closed with suitable Luer locks (Figure 2-VII).
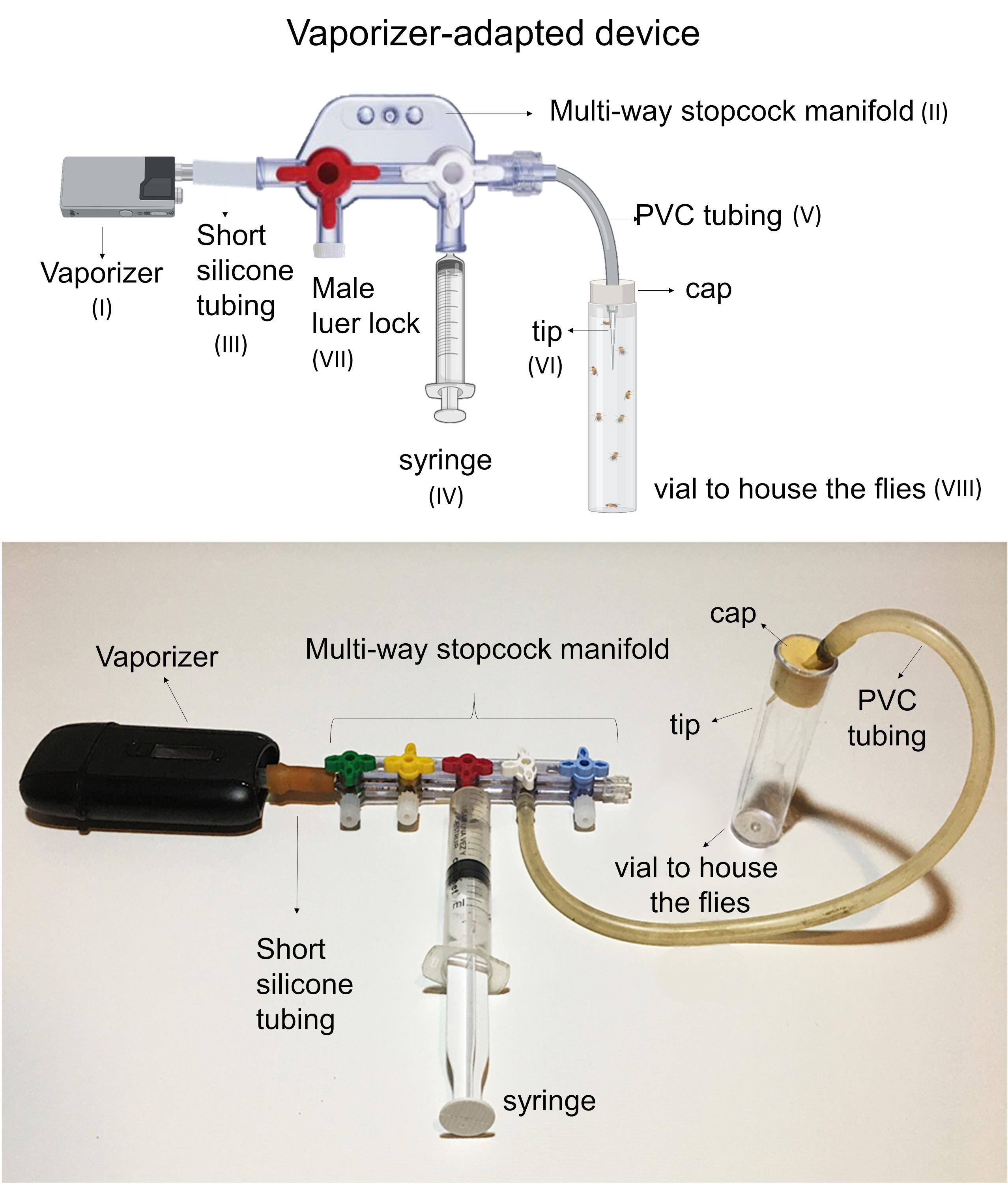
Figure 2. Assembly scheme of the vaporizer-adapted device.Cannabis vaporization procedure (Figure 3 and Video 1)
Transfer the flies from the food vial to an empty vial (Figure 3-VIII) and cover it with a plug (use the same empty vial or another one of a similar size for all experiments).
Grind the vegetal specimen and transfer it into the vaporizer chamber.
Connect the mouthpiece of the vaporizer (Figure 3-I) to the manifold (Figure 3-II).
Insert the free end of the tube attached to the device into the plug of the vial containing flies that will be exposed to vapor (Figure 3-VIII).
Heat up the vaporizer. A dry cannabis vaporizer needs a little time to heat up before you can begin using it. This may take from 10-15 s to over a minute, depending on the size and features of the device.
Ensure the syringe’s plunger (Figure 3-IX) is pressed down to the bottom of the barrel.
Rotate the stopcock connecting the vaporizer to the syringe (Figure 3-X) to allow the vapor to flow from the vaporizer into the syringe.
Pull the plunger back until the desired mark (i.e., desired volume of air). The vapor will flow into the syringe until the desired mark is filled with vapor (Figure 3-step 1).
Rotate the stopcock connecting the vaporizer to the syringe to the closed position in order to prevent more vapor from transferring into the manifold.
Open the valve (Figure 3-XI) that connects the manifold to the vial containing the flies.
Push the plunger of the syringe.
Once the vapor reaches the vial containing the flies, close the valve connecting the manifold to the flies vial (Figure 3-step 2).
Wait the predefined time to ensure the gaseous exchange of vaporized substances across the cuticle and the tracheal system of the flies.
Repeat as many times as needed according to the protocol.
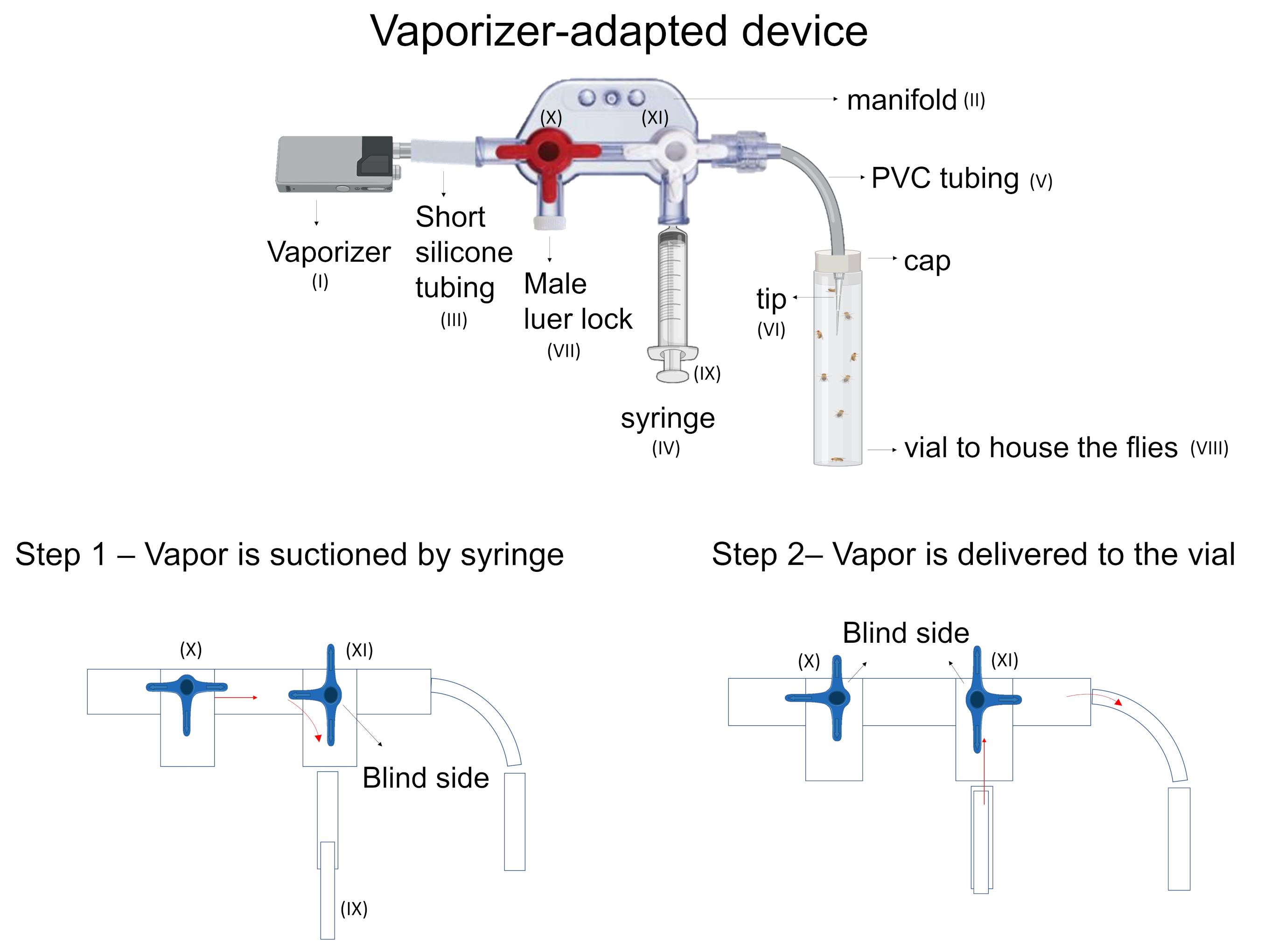
Figure 3. Steps to administer vapor using the vaporizer-adapted device.Video 1. Cannabis and cigarette smoke delivery toDrosophila melanogaster .
Administration of cigarette smoke
Assembly and setting up of the device (Figure 4)
Attach one cigarette (Figure 4-XII) or the filter (Figure 4-XIII) (in case it should be necessary) to the device by connecting it to a stopcock located at one end of the manifold (Figure 4-II). A short silicone tube (Figure 4-III) might be used to connect properly the cigarette or the filter to the end of the manifold.
Connect the aspirator (Figure 4-XIV) contained into the box (Figure 4-XV) to the adjacent stopcock. This is necessary to maintain the cigarette burning.
Connect the syringe (Figure 4-IV) to the next stopcock.
Connect one end of the PVC tubing (Figure 4-V) to the last stopcock, leaving the opposite end free.
Attach a 100 µl tip (Figure 4-VI) to the free end of the PVC tubing.
In case the manifold contains extra valves (i.e., three- or four-way stopcock manifold), they must remain closed with suitable Luer locks.
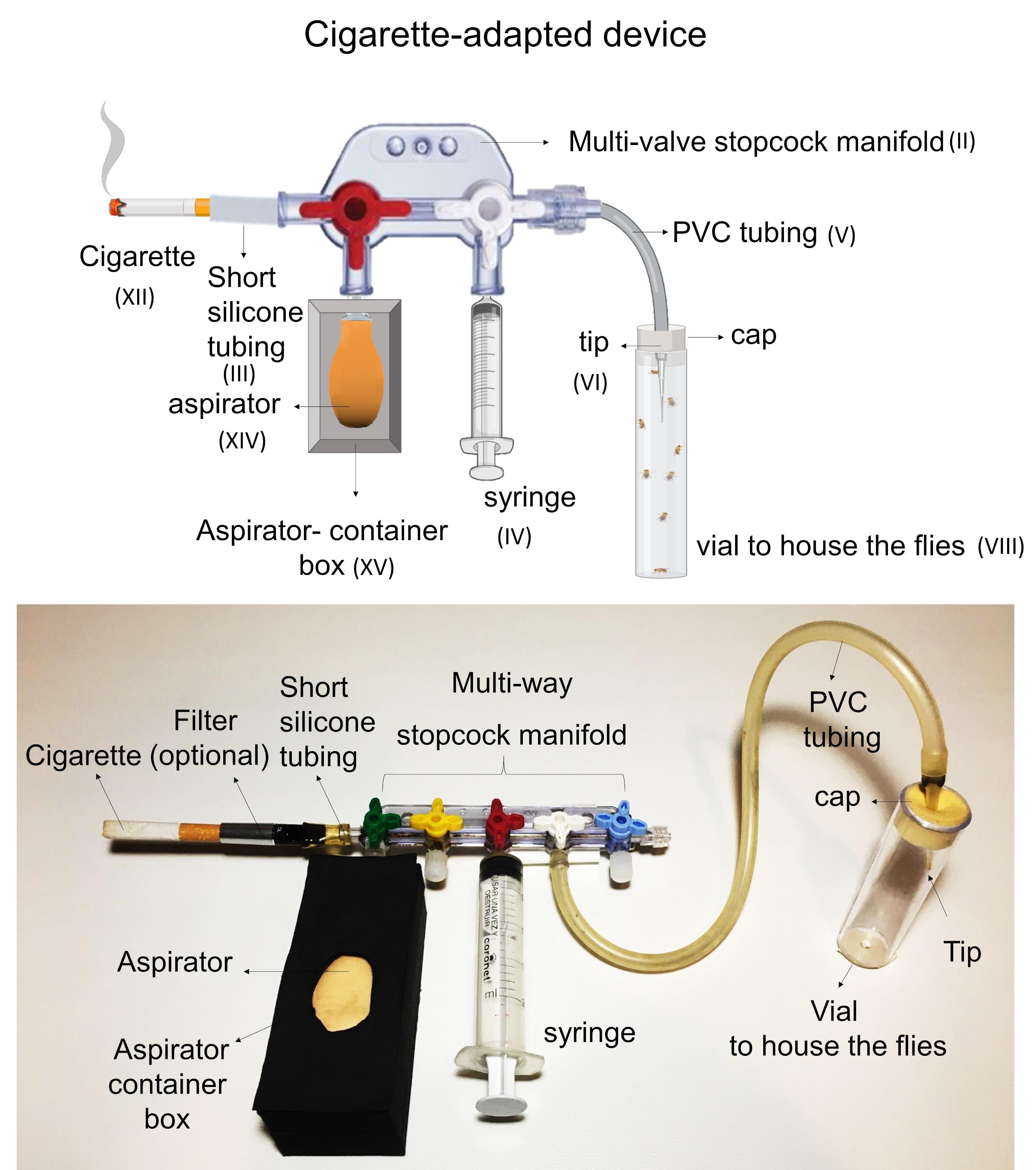
Figure 4. Assembly scheme of the cigarette-adapted device.Smoke administration procedure (Figure 5 and Video 1)
Transfer the flies from the food vial to an empty vial (Figure 5-VIII) and cover it with a plug (use the same empty vial or another one of a similar size for all experiments).
Insert the free end of the tube attached to the device into the plug of the vial (Figure 5-VIII) containing flies that will be exposed to smoke.
Light up the cigarette (Figure 5-XII) and press the aspirator (Figure 5-XIV) to keep the cigarette burning (Figure 5-step 1).
Ensure the syringe’s plunger (Figure 5-IX) is pressed down to the bottom of the barrel.
Rotate the stopcock connecting the cigarette to the syringe (Figure 5-X) to allow the smoke to flow from the cigarette into the syringe.
Pull the plunger back until the desired mark (i.e., the desired volume of air). The smoke will flow into the syringe until the desired mark is filled with smoke (Figure 5-step 2).
Rotate the stopcock connecting the cigarette and the syringe to the closed position to prevent more smoke from being transferred into the manifold.
Open the valve (Figure 5-XI) that connects the manifold to the vial containing the flies.
Push the plunger of the syringe (Figure 5-step 3).
Once the smoke reaches the vial containing the flies, close the valve that connects the manifold to the flies vial.
Wait the predefined time to ensure the gaseous exchange of administrated smoke across the cuticle and the tracheal system of the flies.
Repeat as many times as needed according to the protocol.
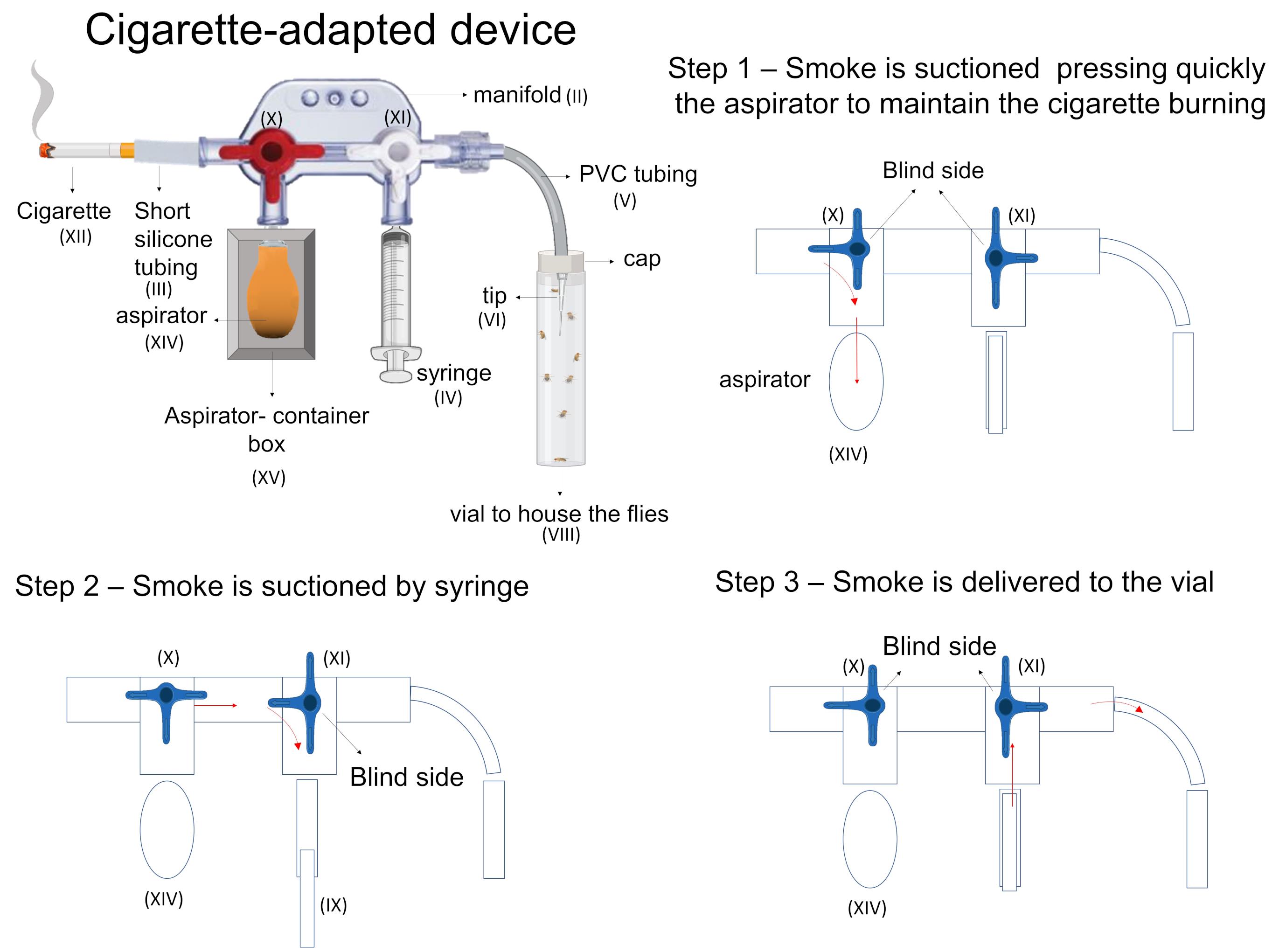
Figure 5. Steps to administer smoke using the cigarette-adapted device.
Data analysis
The protocol described herein is useful for various analyses to test the effects of substances administrated by the inhalation route. In our previously published articles, we focused our studies on the cardiac performance of wild-type adult individuals of Drosophila, as well as in strains containing specific gene mutations (Gómez et al., 2019; Santalla et al., 2021).
Providing vaporized cannabis extract to adult flies did not modify heart rate, among other parameters (Gómez et al., 2019), whereas exposure to a commercial cigarette (Philip Morris) increased heart rate (Santalla et al., 2021).
This reveals that different substances administered by means of the same device, suitably adapted to the corresponding substance source (dry cannabis or a commercial cigarette), can be analyzed according to the respective experimental procedures.
Although our vaporizer is available only for cannabis, the custom device is useful for extracts such as nicotine extract/juice, among others. The user should select a suitable vaporizer for the particular extract, as indicated in the equipment section.
Independently of the focus of study, this technique might allow testing different aspects of fly behavior, i.e., sleep, mating, feeding, and survival, as well as physiological parameters, biochemical and molecular mechanisms in wild-type and/or mutant strains. The simplicity of the setup might allow its implementation at a low cost for research and/or educational purposes.
Notes
Electronic cigarette
In case the user utilizes an electronic cigarette instead a disposable cigarette, preparation of the extract should be made according to the solubility of the substance of interest.
Combination of different substances
Depending on the test substance and the temperature of vaporization for each compound, flies can be exposed to different substances during the same experiment by regulating the temperature of the vaporizer. For example, when using Cannabis sp., a fraction of terpenes and cannabinoids are vaporized together. Combination of pure drugs is also possible.
Final considerations: Device maintenance and estimated cost of assembly
The manifold should be cleaned regularly, according to the frequency of use. We recommend cleaning it after 10 assays by means of a thin bottle brush with water and a commercial non-ionic detergent, followed by a rinse with ultrapure water. Silicone and PVC tubing should be replaced when the user observes increased opacity of the walls after repeated uses. Drosophila vials should be replaced periodically. Vaporizer or electronic cigarettes should be cleaned according to the manufacturer's instructions. Disposable cigarettes should be bought according to the frequency of use.
The estimated cost of the entire device is approximately USD 200. The vaporizer is the most expensive component, followed by the electronic cigarette. Tubing is usually sold as 15 meters roll, although a smaller length might be acquired.
Recipes
Fly food for stocks (1.5 L)
107.25 g of glucose
21.45 g of yeast
12.9 g of agar
121.5 g of yellow cornmeal
1462.5 ml of tap water
37.5 ml of 10% w/v Nipagin in ethanol
Note: Stir and heat all the ingredients except Nipagin until the mixture reaches 100°C. Remove from heat and allow warming for 20 min. Add 10% w/v Nipagin in ethanol (3.75 g of Nipagin in 37.5 ml of ethanol), stir, and allow warming again. Pour into fly vials, cover with cotton and let dry. Store vials containing food in a Drosophila incubator at 17°C.
Cannabis treatment
Dried cannabis inflorescences must be minced with scissors into very small pieces. A grinder can also be used for this process. A desired amount of minced cannabis (e.g., 0.03 g) must be placed inside the vaporizer, which is set to 180°C to allow the vaporization of cannabis compounds.
Acknowledgments
This work was supported by ANPCyT PICT 2014-2549 and SIB 2019-0606 UNNOBA to PF.
Previous works: Gómez et al., 2019; Santalla et al., 2021 .
Competing interests
The authors declare no competing financial and non-financial interests.
References
- Bainton, R. J., Tsai, L. T., Singh, C. M., Moore, M. S., Neckameyer, W. S. and Heberlein, U. (2000). Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol 10(4): 187-194.
- Baker, B. M., Carbone, M. A., Huang, W., Anholt, R. R. H. and Mackay, T. F. C. (2021). Genetic basis of variation in cocaine and methamphetamine consumption in outbred populations of Drosophila melanogaster. Proc Natl Acad Sci U S A 118(23): e2104131118.
- Broderick, K. E., Potluri, P., Zhuang, S., Scheffler, I. E., Sharma, V. S., Pilz, R. B. and Boss, G. R. (2006). Cyanide detoxification by the cobalamin precursor cobinamide. Exp Biol Med (Maywood) 231(5): 641-649.
- Chvilicek, M. M., Titos, I. and Rothenfluh, A. (2020). The Neurotransmitters Involved in Drosophila Alcohol-Induced Behaviors. Front Behav Neurosci 14: 607700.
- Filošević, A., Al-Samarai, S. and Andretic Waldowski, R. (2018). High Throughput Measurement of Locomotor Sensitization to Volatilized Cocaine in Drosophila melanogaster. Front Mol Neurosci 11: 25.
- Gómez, I. M., Rodriguez, M. A., Santalla, M., Kassis, G., Colman Lerner, J. E., Aranda, J. O., Sedan, D., Andrinolo, D., Valverde, C. A. and Ferrero, P. (2019). Inhalation of marijuana affects Drosophila heart function. Biol Open 8(8): bio044081.
- Linde, K. van der, Fumagalli, E., Roman, G., Lyons, L.C. (2014). The FlyBar: Administering Alcohol to Flies. J Vis Exp 87: 50442.
- McClung, C. and Hirsh, J. (1998). Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr Biol 8(2): 109-112.
- Morris, M., Shaw, A., Lambert, M., Perry, H. H., Lowenstein, E., Valenzuela, D. and Velazquez-Ulloa, N. A. (2018). Developmental nicotine exposure affects larval brain size and the adult dopaminergic system of Drosophila melanogaster. BMC Dev Biol 18(1): 13.
- Pandey, U. B. and Nichols, C. D. (2011). Human disease models in drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev 63(2): 411-436.
- Park, A., Tran, T., Gutierrez, L., Stojanik, C. J., Plyler, J., Thompson, G. A., Bohm, R. A., Scheuerman, E. A., Smith, D. P. and Atkinson, N. S. (2021). Alcohol-induced aggression in Drosophila. Addict Biol: e13045.
- Petruccelli, E., Feyder, M., Ledru, N., Jaques, Y., Anderson, E. and Kaun, K. R. (2018). Alcohol Activates Scabrous-Notch to Influence Associated Memories. Neuron 100(5): 1209-1223 e1204.
- Rigo, F., Filosevic, A., Petrovic, M., Jovic, K. and Andretic Waldowski, R. (2021). Locomotor sensitization modulates voluntary self-administration of methamphetamine in Drosophila melanogaster. Addict Biol 26(3): e12963.
- Sanchez-Díaz, I., Rosales-Bravo, F., Reyes-Taboada, J. L., Covarrubias, A. A., Narvaez-Padilla, V. and Reynaud, E. (2015). The Esg Gene Is Involved in Nicotine Sensitivity in Drosophila melanogaster. PLoS One 10(7): e0133956.
- Santalla, M., Pagola, L., Gomez, I., Balcazar, D., Valverde, C. A. and Ferrero, P. (2021). Smoking flies: testing the effect of tobacco cigarettes on heart function of Drosophila melanogaster. Biol Open 10(2).
- Shohat-Ophir, G., Kaun, K. R., Azanchi, R., Mohammed, H. and Heberlein, U. (2012). Sexual deprivation increases ethanol intake in Drosophila. Science 335(6074): 1351-1355.
- Tatum-Gibbs, K. R., McKee, J. M., Higuchi, M. and Bushnell, P. J. (2015). Effects of toluene, acrolein and vinyl chloride on motor activity of Drosophila melanogaster. Neurotoxicol Teratol 47: 114-124.
- Velazquez-Ulloa, N. A. (2017). A Drosophila model for developmental nicotine exposure. PLoS One 12(5): e0177710.
- Wilson, M., Widdicombe, J. H., Gohil, K., Burtis, K. C., Reznick, A. Z., Cross, C. E. and Eiserich, J. P. (2005). Are Drosophila a useful model for understanding the toxicity of inhaled oxidative pollutants: a review. Inhal Toxicol 17(13): 765-774.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Santalla, M., Gómez, I. M., Valverde, C. A. and Ferrero, P. (2021). A low-cost Portable Device to Deliver Smoke, Volatile or Vaporized Substances to Drosophila melanogaster , Useful for Research and/or Educational Assays. Bio-protocol 11(23): e4244. DOI: 10.21769/BioProtoc.4244.
Category
Biological Engineering > Biomedical engineering
Drug Discovery
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











