- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determination of Fungal Tolerance Index to Heavy Metals and Heavy Metal Resistance Tests
Published: Vol 11, Iss 21, Nov 5, 2021 DOI: 10.21769/BioProtoc.4218 Views: 2903
Reviewed by: Alexandros AlexandratosElías Barquero-CalvoAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
An Assay to Determine NAD(P)H: Quinone Oxidoreductase Activity in Cell Extracts from Candida glabrata
Anamika Battu [...] Rupinder Kaur
Nov 5, 2021 3051 Views
High-throughput Growth Measurements of Yeast Exposed to Visible Light
Katarina Logg [...] Mikael Molin
Jan 20, 2022 3174 Views
Immunoisolation of Endosomal Recycling Vesicles from Saccharomyces cerevisiae
Sho W. Suzuki and Scott D. Emr
May 5, 2022 2716 Views
Abstract
Fungal metallo-tolerance has been described in different species and plays an important role in bioremediation of contaminated environments. Metallo-tolerance is mainly documented by microdilution assays and agar well diffusion methods using equipment that can be expensive. The tolerance index can be calculated to determine the efficiency of a fungus to degrade and resist heavy metals. The present protocol is based on analyzing the tolerance index and minimum inhibitory concentration of the metallo-tolerance potential of culturable fungi on solid media. This can be calculated by daily measurements of colony size on agar supplemented with different concentrations of heavy metals. This method is an easy approach to determine fungal heavy metal resistance using simple laboratory equipment without spectroscopy.
Keywords: Heavy metalsBackground
The increasing use of large amounts of toxic heavy metals in industrial processes, lack of proper disposal techniques, and their non-degradable nature is creating problems to the environment and in human health (Akhtar et al., 2013; Kumar et al., 2014). Some fungi have been isolated from anthropogenic contaminated sites and tolerate high amounts of toxic compounds (Rehman and Anjum, 2011). Furthermore, they can absorb, accumulate, and resist heavy metals (Fernández et al., 2017). Therefore, it is useful to describe protocols based on metallo-tolerant fungal capacity that serve as agents in the bioremediation process. One of the most common methodologies to determine heavy metal resistance is microdilution using flasks or polystyrene microtiter plates, broths, or synthetic culture media (RPMI 1640 medium), followed by spectrophotometry (Al-janabi, 2010). However, this method needs specific equipment and could be expensive for researchers from developing countries like Mexico (Calvillo-Medina et al., 2020). The agar dilution method proposed here works better for filamentous fungi and is an easy option to evaluate heavy metal fungal resistance (Iram et al., 2012). Based on this evidence, we reported a method to evaluate heavy metal resistance and to determine fungal tolerance index (TI), which could be calculated to identify the most tolerant microorganisms even with fastidious isolates. TI calculations can be used without agar dilution methods, e.g., when fungal isolates grow according to the microdilution method or the growth of filamentous bacteria in the presence of toxic compounds.
Materials and Reagents
Petri dish 100 mm diameter (SYM Laboratorios, catalog number: 7730SYM)
Pipette tips (Thermo Scientific, catalog numbers: 94300210 [1,000 μl]; 94300110 [100 μl] and 101-96R-Q [10 μl])
5 mm diameter sterile stainless steel punch (Avantor, catalog number: 10194-928)
0.22 μm sterile filters (Millipore, catalog number: SLGVV255F)
5 ml syringe for filter sterilization (JTC, catalog number: 05LSA-NPRO)
15 ml tubes (Axygen, catalog number: SCT-15ML-500)
Caliper (MIYUTOYO, catalog number: 531)
Erlenmeyer flask (Pyrex, catalog number: CLS44501L-1EA)
Fungal isolates to test
Culture media Potato dextrose agar (PDA) (Bioxon, catalog number: 210700)
Distilled water (Meyer, catalog number: HDS 0345)
Selected heavy metals, used as salts:
Chromium Cr(III) (CrCl3) (Sigma-Aldrich, catalog number: 450790)
Mercury Hg(II) (HgCl2) (Sigma-Aldrich, catalog number: 215465)
Lead Pb(II) (Pb(C2H3O2)2) (Meyer, catalog number: HDS 1955)
Equipment
Analytical balance (Ohaus Adventurer, catalog number: H-5276)
Pipettes (Thermo Scientific, catalog numbers: 4651270N [1,000 μl]; 4651230N [100 μl] and 4651280N [10 μl])
Autoclave (Evar, catalog number: EV-24)
Microbiological incubator (Thermo Fisher Scientific, catalog number: 50129111)
Microbiological safety cabinet (Thermo Fisher Scientific, catalog number: 1310TS1)
Software
T-test package ggpubr (R package version 0.1.7. https://CRAN.R-project.org/package=ggpubr)
R Core Team (https://www.Rproject.org/)
Procedure
Heavy metal stocks and culture media
Prepare stocks under sterile conditions in six different concentrations: 2.5 mM, 5 mM, 7.5 mM, 10 mM, 15 mM, and 20 mM of Cr(III), Hg(II), and Pb(II).
Dissolve the meal salts for each concentration in distilled water, filter sterilize using 0.22 µm filters, and store at room temperature until use (up to 3 months).
Prepare PDA solid media in Erlenmeyer flasks using distilled water and sterilize by autoclaving (121°C and 15 psi pressure for 20 min). One flask of agar per heavy metal concentration and one without heavy metal solutions, to use as a control for each fungal isolate, are needed.
After sterilization, let the agar cool at 50°C and add the heavy metal solutions under sterile conditions. Pour 20 ml of supplemented agar per Petri dish. Store at 4°C until use (up to 3 months).
Cultivation methods
Under sterile conditions, inoculate fungal isolates (1 × 106 conidia/ml) on PDA without heavy metal supplementation and incubate for 14 days at 28°C to induce conidiation (pre-grown state).
Punch out agar blocks of fresh mycelium from the edge of the colony using a 5 mm diameter sterile stainless steel punch after the first incubation time (pre-grown state). This step should be performed in the microbiological safety cabinet. Several plugs per strain are necessary depending on the number of plates with different heavy metal concentrations.
Inoculate the plates with different concentrations of heavy metals and the control in the center with one plug per plate and incubate at 28°C for 14 days.
Six replicates should be done for each fungus and heavy metal concentration and for control cultures. Representative fungal growth of two isolates is displayed in Figure 1.
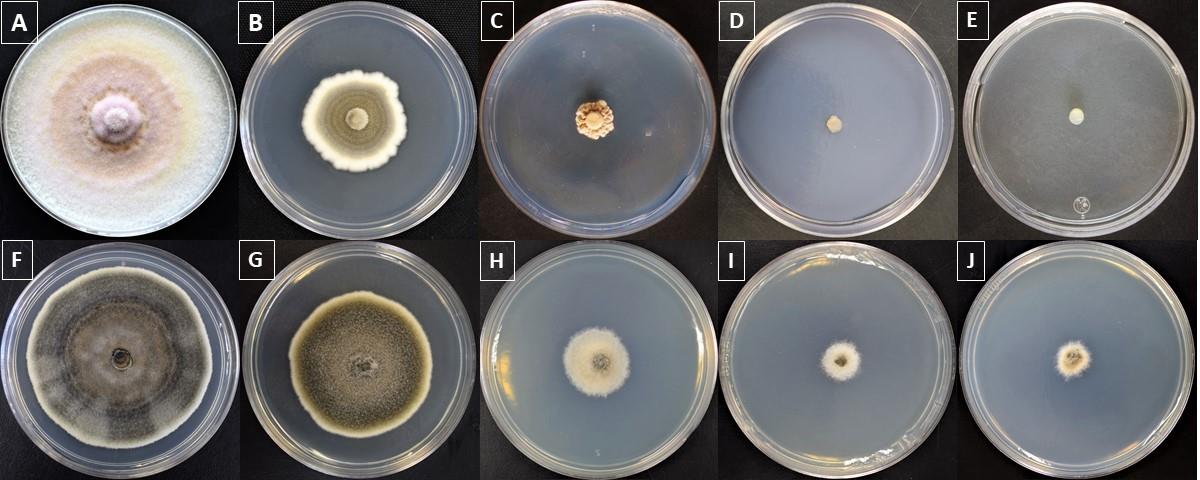
Figure 1. Fungal heavy metal resistance at four different concentrations after 14 days of incubation on PDA. The figure displays Periconia citlaltepetlensis (top) and Alternaria sp. growth (bottom) without heavy metals (A, F) and in the presence of Cr(III) at 2.5 mM (B, G), 5 mM (C, H), 7.5 mM (D, I), or 10 mM (E, J).
Minimum inhibitory concentrations and tolerance index
After inoculation, perform daily measurements of colony size with caliper.
Calculate the radial growth based on five measurements (in millimeters) during 14 days of growth by subtracting the initial diameter (5 mm) of the plug (Figure 2 and Table 1).
Data analysis
MIC is defined as the minimum concentration that inhibited the visible growth of isolates after 14 days (Joo and Hussein, 2012).
Results are graphed and statistically evaluated with the unpaired two-samples Student's t-test using T-test package ggpubr (Kassambara, 2018) in R (R Core Team, 2018). Statistics and graphs will help to understand the capacity to tolerate metals of each fungus tested (Figure 2).
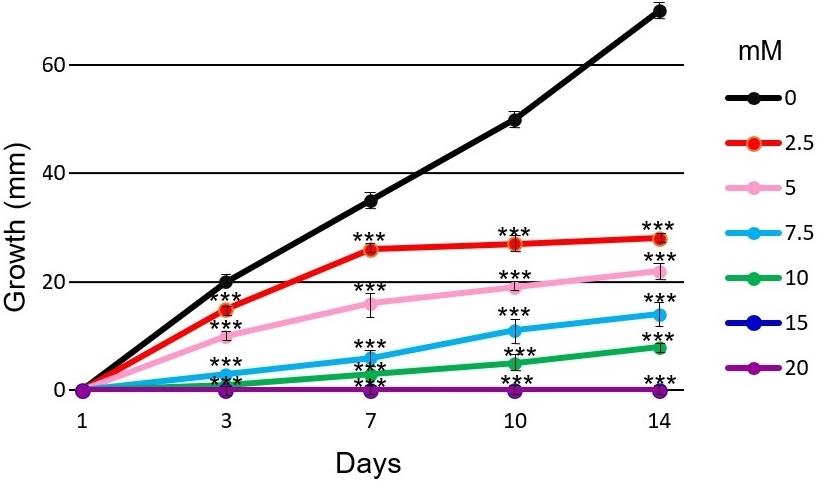
Figure 2. Effect of different concentrations of lead on Alternaria sp. growth. The fungus was cultured at 28°C for 14 days on PDA agar at six different concentrations of Pb(II) (2.5 mM, 5 mM, 7.5 mM 10 mM, 15 mM, and 20 mM). Error bars represent the standard error of the mean of three technical replicates in at least two independent measurements.of each incubation period. n = 5. ***, P-value < 0.001 for fungal growth on lead compared with the control.The TI calculation, which is an indication of how an organism tolerates stress (Mohammadian-Fazli et al., 2015), is determinate for each metal and fungal strain with the daily measurements of colonies (Figure 3). TI should be calculated based on measurements of heavy metal fungal growth (HMFG) at different concentrations divided by control culture fungal growth (CFG).
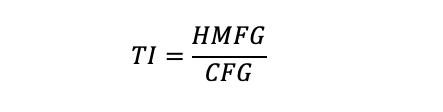
A TI close to 1 indicates that the fungus is resistant to the tested heavy metal concentration (Figure 3).
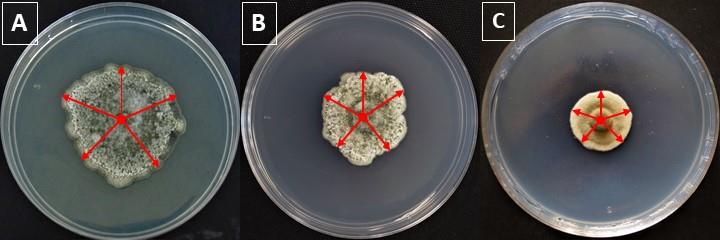
Figure 3. Measurements of Curvularia sp. at two different concentrations of chromium. The figure shows control fungal growth (CFG) (A) and on 2.5 mM (B) or 5 mM (C) of CrCl3 (HMFG). Red arrows represent daily measurements to monitor fungal growth.To select the most tolerant fungi, the values of TI and MIC assays must be assessed. Representative data of MIC and TI values of three isolates are displayed in Table 1.
Table 1. MIC and TI of three extremophilic fungi at different concentrations of Cr(III), Hg(II), and Pb(VI) growth on media for 14 days
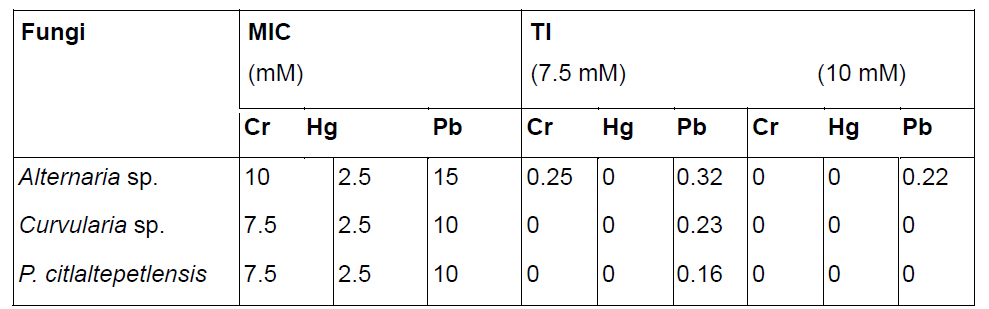
MIC: Minimum inhibitory concentrations in millimolar.
TI: Tolerance index calculation at 7.5 mM and 10 mM of heavy metals.
Notes
This is a simple, easier, and cheaper method to generate relevant data about metallo-tolerance and a possible bioremediation process.
The media (PDA) proposed here is useful with a broad range of fungal species, even those which are difficult to culture.
Fastidious isolates (e.g., extremophilic and psychrotolerant fungi) were successfully tested with this protocol.
Acknowledgments
The author thanks CONACYT Postdoctoral scholarship (005352) and appreciates the collaboration of Fernández-Tellez IE in Figure 1. This work is dedicated to empowered women who climb glaciers, fight against misogyny, and for equality in Mexico. This protocol is based on the original research paper (Calvillo-Medina et al., 2020).
Competing interests
The author declares that there are no conflicts of interest.
References
- Akhtar, S., Mahmood-ul-Hassan, M., Ahmad, R., Suthor, V., and Yasin, M. (2013). Metal tolerance potential of filamentous fungi isolated from soils irrigated with untreated municipal effluent. Soil Environ 32(1): 55-62.
- Al-janabi, A. A. H. S. (2010). Toxic effect of heavy metals on dermatophytes. Mycoses 54: 345-349.
- Calvillo-Medina, R. P., Gunde-Cimerman, N., Escudero-Leyva, E., Barba-Escoto, L., Fernández-Tellez, E. I., Medina-Tellez, M. M., Bautista-de, Lucio, V., Ramos-López, M. A. And Campos-Guillén, J. (2020). Richness and metallo-tolerance of cultivable fungi recovered from three high altitude glaciers from Citlaltépetl and Iztaccíhuatl volcanoes (Mexico). Extremophilles 24(4): 625-636.
- Fernández-Marcelo, P., Martorell, M. M., Blaser, M. G., Mauro-Ruberto, L. F., Castellanos-de, Figueroa, L. I. and Mac Cormack, W. P. (2017). Phenol degradation and heavy metal tolerance of Antarctic yeasts. Extremophiles 21: 445-457.
- Iram, S., Parveen, K., Usman, J., Nasir, K., Akhtar, N., Arouj, S. and Ahmad, I. (2012). Heavy metal tolerance of filamentous fungal strains isolated from soil irrigated with industrial wastewater. Biologija 58(3): 107-116.
- Joo, J. H. and Hussein, K. A. (2012). Heavy metal tolerance of fungi isolated from contaminated soil. Korean J Soil Sci Fert 45(4): 565-571.
- Kassambara, A. (2018). ggpubr: ’ggplot2’ Based Publication Ready Plots. R package version 0.1.7.
- Kumar, R., Singh, P., Dhir, B., Sharma, K. A. and Mehta, D. (2014). Potential of some fungal and bacterial species in bioremediation of heavy metals. J Nuclear Physics, Mater Sci, Radiat Appl 1(2): 213-223.
- Mohammadian-Fazli, M. M., Soleimani, N., Mehrasbi, M., Darabian, S., Mohammadi, J. and Ramazani, A. (2015). Highly cadmium tolerant fungi: their tolerance and removalpotential. J Environ Health Sci Eng 13: 19.
- R Core Team. (2018). R: A Language and environment for statistical computing. R Foundation for Statistical Computing.
- Rehman, A. and Anjum, M. S. (2011). Multiple metal tolerance and biosorption of cadmium by Candida tropicalis isolated from industrial effluents: Glutathione as detoxifying agent. Environ Monit Assess 174(1-4): 585-595.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Calvillo-Medina, R. P. (2021). Determination of Fungal Tolerance Index to Heavy Metals and Heavy Metal Resistance Tests. Bio-protocol 11(21): e4218. DOI: 10.21769/BioProtoc.4218.
Category
Microbiology > Microbial physiology > Stress response
Biological Sciences > Microbiology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









