- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Simple Methods for the Preparation of Colloidal Chitin, Cell Free Supernatant and Estimation of Laminarinase
Published: Vol 11, Iss 19, Oct 5, 2021 DOI: 10.21769/BioProtoc.4176 Views: 5149
Reviewed by: Khyati Hitesh ShahXin XuChhuttan L Meena

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Monitoring Protein Stability In Vivo Using an Intein-Based Biosensor
John S. Smetana [...] Christopher W. Lennon
Apr 20, 2025 1571 Views
Surface Plasmon Resonance for the Interaction of Capsular Polysaccharide (CPS) With KpACE
Zhe Wang [...] Chao Cai
Jun 20, 2025 3552 Views
Thermus thermophilus CRISPR Cas6 Heterologous Expression and Purification
Junwei Wei [...] Yingjun Li
Jul 20, 2025 2159 Views
Abstract
Colloidal chitin (CC) is a common substrate used in research work involving chitin-active enzymes (chitinases). Cell free supernatant (CFS) is prepared from fermented broth. Preparation of CC and CFS usually involve large amounts of liquid, which must be separated from the solids. This necessitates the use of a large volume centrifugation facility, which may not be accessible to everyone. Filtration is a viable alternative to centrifugation, and several filter elements are described in the literature. Each of those elements has its own set of disadvantages like non-availability, high cost, fragility, and non-reusability. Here we describe the use of lab coat clothing material (LCCM) for the preparation of CC and CFS. For filtration purposes, the LCCM was found to be functional, rugged, reusable, and cost-effective. Also described here is a new method for the estimation of laminarinase using a laminarin infused agarose gel plate. An easily available optical fabric brightener (OFB) was used as a stain for the agarose plate. The laminarin infused agarose plate assay is simple, inexpensive, and was found to be impervious to high amounts of ammonium sulfate (AS) in enzyme precipitates.
Keywords: Colloidal chitinBackground
Separation of insoluble solids from liquids may be achieved by either centrifugation or filtration. The use of centrifugation for the preparation of colloidal chitin (CC) and cell free supernatant (CFS) has limitations as a large volume of liquids are involved (Hsu and Lockwood, 1975; Mitsutomi et al., 1995). Preparation of CFS from lab-scale and pilot-scale fermented broth is particularly challenging (Sarkar et al., 2010). Thus, a large capacity centrifuge machine (preferably refrigerated) with suitable rotor must be used (e.g., Sorvall BIOS 16). These facilities may not be available to researchers, especially in developing countries. Under these circumstances, filtration can be a viable alternative to centrifugation.
Most of the filter elements described in the literature (viz. filter paper, paper coffee filter, Miracloth, cheese cloth, etc.) suffer from one or more of the drawbacks listed below (Sandhya et al., 2004; Javaid and Ali, 2011; Murthy and Bleakley, 2012; Nidheesh and Suresh, 2015; Park et al., 2015): (1) Expensive (Miracloth); (2) Fragile (filter paper and paper coffee filter); (3) Non-reusable (filter paper and paper coffee filter); (4) Poor filtration rate (cheese cloth, filter paper, and paper coffee filter) and (5) Not readily available (paper coffee filter and Miracloth).
In this work, a 280 g per square meter (GSM) 65/35 polyester-cotton blend LCCM was used as a filter element. LCCM was found to be perfectly suitable for the preparation of CC. CFS was prepared from Streptomyces rimosus AFM-1 using a similar set of materials and is most suitable for organisms exhibiting pellet growth (viz. fungi and actinomycetes) (Koteshwara et al., 2021).
Laminarin (β(1→3)-glucan interspersed with β(1→6)-branches) is the long-term carbon storage polymer of glucose found in brown algae (Beattie et al., 1961; Graiff et al., 2016). Laminarinases ((1→3)-β-glucan hydrolase) are a type of hemicellulase that act upon and hydrolyze laminarin. They can be classified as either endo-type (EC 3.2.1.39) or exo-type (EC 3.2.1.58) based on the substrate hydrolysis pattern (Wu et al., 2018). The laminarinase enzyme is produced by a wide variety of organisms and plays an important role in natural ecosystems for the recycling of carbon (Graiff et al., 2016). Bacteria usually only transport substrates with a molecular mass of <600Da (Weiss et al., 1991; Grinter and Lithgow, 2020). Consequently, many marine bacteria produce extracellular enzymes like laminarinases to hydrolyze high molecular mass substrates (e.g., laminarin: 2,000-7,000 Da) (Yang et al., 2020). β-Glucan hydrolases are used in the food industry, medicine, and biotechnology (Qin et al., 2017).
Commercial laminarin used in the laboratory is usually sourced from either Laminaria digitata (Sigma-Aldrich L9634) or Eisenia bicyclis (TCI L0088). Interestingly, laminarin has similarities to the β-glucans found in the fungal cell wall. Thus, it is used to evaluate the anti-fungal β-glucan hydrolases produced by various organisms, especially the biocontrol agents (Scott and Schekman, 1980; Watanabe et al., 1989; De la Cruz et al., 1995; Aktuganov et al., 2008). Under these circumstances, detection and estimation of laminarinase enzyme assumes importance.
AS precipitation continues to be the most preferred method for the separation of proteins from CFS due to the following reasons (Wingfield, 2001; Duong-Ly and Gabelli, 2014): (1) It is inexpensive, simple, germicidal, highly soluble in water, and exerts a stabilizing effect on the precipitated proteins; (2) For the precipitation of native and non-recombinant proteins; (3) It can inhibit proteolytic activity; and (4) Precipitation of recombinant protein without an affinity tag or an unexposed affinity tag.
AS precipitation is a non-specific method. Thus, optimization of the AS concentration must be carried out to reduce the amount of contaminating proteins. AS interferes with most enzyme assays, thus the precipitates must be dialyzed before evaluation. A simple and rapid assay that provides results without the need for dialysis would ease the AS optimization task; this idea was the motivation for this research work (Koteshwara et al., 2021).
Plate assays for enzymes are inherently much simpler than liquid assays. This is due to their simple design, ability to evaluate multiple samples in a single plate, and the fact that they provide direct qualitative/ semi-quantitative results (Teather and Wood, 1982; Samad et al., 1989; Gulati et al., 1997; Meddeb-Mouelhi et al., 2014; Sawant et al., 2015; Patil and Chaudhari, 2017). We have developed a plate assay for laminarinase using a laminarin-infused agarose gel. Laminarin is a translucent substrate necessitating the use of a dye for the detection of zones of hydrolysis. An easily available OFB Tinopal CBS-X (Disodium 4,4'-bis(2-sulfostyryl) biphenyl) was tested as a stain and found suitable. The plate assay provided accurate, reproducible results even in the presence of a high concentration of AS (Koteshwara et al., 2021). Congo red is an extensively used dye for the detection of polysaccharide hydrolysis but is not recommended for use with laminarin gel plate assay due to poor contrast (Wood et al., 1988). Many wide-ranging and complicated methods can be found in the literature for the preparation of CC, CFS, and laminarinase plate assay. Simple alternatives to complex protocols are described here.
Materials and Reagents
Glass funnel plain (60° Angle and short stem) (Borosil, catalog number: 6140077)
Petri dishes (3.9” and 5.9” diameters) (Borosil, catalog numbers: 3165A77 [3.9”]; 3165081 [5.9”])
Conical flask with screw cap (250 ml capacity recommended) (Borosil, catalog number: 5021024)
Plastic tray (preferably polypropylene) (Tarsons, catalog number: 240090)
Cellulose membrane dialysis tubing (14 kDa cutoff) (Sigma-Aldrich, catalog number: D9652)
Steam indicator tape (3M, catalog number: 1322)
Dry heat indicator strip (HiMedia Laboratories, catalog number: LA812)
Laminarin derived from Laminaria digitata (Sigma-Aldrich, catalog number: L9634)
Shrimp chitin powder (HiMedia Laboratories, catalog number: RM1356)
(NH4)2SO4 (HiMedia Laboratories, catalog number: GRM1192)
KH2PO4 (HiMedia Laboratories, catalog number:GRM2951)
K2HPO4 (HiMedia Laboratories, catalog number: GRM168)
Yeast extract powder (HiMedia Laboratories, catalog number: RM027)
Malt extract powder (HiMedia Laboratories, catalog number: RM004)
Glucose (HiMedia Laboratories, catalog number: GRM016)
NaCl (HiMedia Laboratories, catalog number: GRM3954)
Agarose (HiMedia Laboratories, catalog number: MB002)
MgSO4·5H2O (Sisco Research Laboratories, catalog number: 85611)
FeSO4·7H2O (Sisco Research Laboratories, catalog number: 97868)
ZnSO4·5H2O (Sisco Research Laboratories, catalog number: 35243)
Tinopal CBS-X 99% pure (AksharChem, catalog number: AKSHAR1979)
37% w/w hydrochloric acid (Merck Life Science, catalog number: 1003172011)
65/35 polyester-cotton blend 280 GSM LCCM (generic purchased from local market or collected from an old laboratory coat)
Distilled water (prepared in-house)
Laminarin infused agarose gel (see Recipes)
Tinopal CBS-X OFB staining solution (see Recipes)
Chitin acid slurry (see Recipes)
CCMS broth medium (see Recipes)
Note: All materials were kept at room temperature, protected from excessive humidity.
Equipment
Microwave oven (Samsung, model: MS23F301TAK/TL)
Antibiotic Zonescale (HiMedia Laboratories, catalog number: PW297)
Glazed porcelain or plastic Buchner funnel with fixed perforated plate 6” diameter (Porcelain: Fisher Scientific, FisherbrandTM, catalog number: FB966J; Plastic: Cole-Parmer, DynalonTM, catalog number: WW-06128-10)
Rubber adapter for Buchner funnels (optional for use with vacuum pump) (generic)
Vacuum pump (optional) (Toshniwal Instruments (Madras), model: TDC6)
Magnetic stirrer and round magnetic stirrer bar with pivot ring 8 × 65 mm (REMI Sales and Engineering Ltd., Magnetic stirrer: model: 1ML; Magnetic stir bar: Tarsons, catalog number: 4115)
Autoclave or pressure cooker for sterilization (Autoclave: Osworld, model: JRIC-39; Pressure cooker: TTK Prestige., model: Popular 16L)
Glass stirring rod and plastic spatula
Plastic beaker 2 L with spout and handle (Sigma-Aldrich, BrandTM, catalog number: BR41012)
Buchner flask (>2 L capacity recommended) (Sigma-Aldrich, PyrexTM, catalog number: SLW1170/14D)
Stage micrometer and eyepiece graticule (Erma Inc., model: ESM11)
Microscope (Olympus, model: CX41)
Porcelain mortar and pestle (Sigma-Aldrich, CoorsTM, catalog number: Z247499)
Water distillation system (Borosil, catalog number: 3361-1.5 L)
Software
Java runtime environment (Oracle, https://www.java.com/en/)
Fiji (ImageJ) (Laboratory for Optical and Computational Instrumentation, University of Wisconsin at Madison, USA. https://imagej.net/Welcome)
Procedure
Safety note: Initial steps in the preparation of CC use concentrated mineral acid (HCl). Wearing eye protection device and gloves and following other safety measures are necessary. Perform the steps involving concentrated acid either in a fume hood or in a well-ventilated area.
Preparation of CC and CFS using the LCCM based filtration apparatus
A1. Preparation of CC
Weigh 10 g of shrimp chitin powder in a 250 ml glass beaker and mix it with 100 ml of 37% w/w HCl. Mix it slowly but thoroughly using a glass rod. Make sure there are no clumps of chitin powder. A very thick slurry will form, which slowly turns brown.
Transfer the beaker into a plastic tray filled with chilled water or ice. This will reduce the heat generated due to the addition of the concentrated acid.
Keep mixing the slurry every 5 min with the glass rod for 30 min.
The slurry will become noticeably less viscous during the final stages of this process.
After the acid hydrolysis step, slowly mix the acid slurry with 2 L of chilled distilled water with concomitant mixing. Use a magnetic stirrer for this step. A white fluffy substance will immediately form. Let this suspension mix for at least 15-20 min. Longer mixing times improve the homogeneity of the suspension.
Assemble the LCCM based filtration apparatus as depicted in Figure 1A and Video 1. A single layer of the LCCM is enough to filter the CC. Use rubber bands to tightly hold the LCCM in place. Ensure that most of the surface of the perforated plate of the funnel is in contact with the LCCM. Place the filtration apparatus inside a tray to collect any liquids that may drip from the LCCM due to wicking.
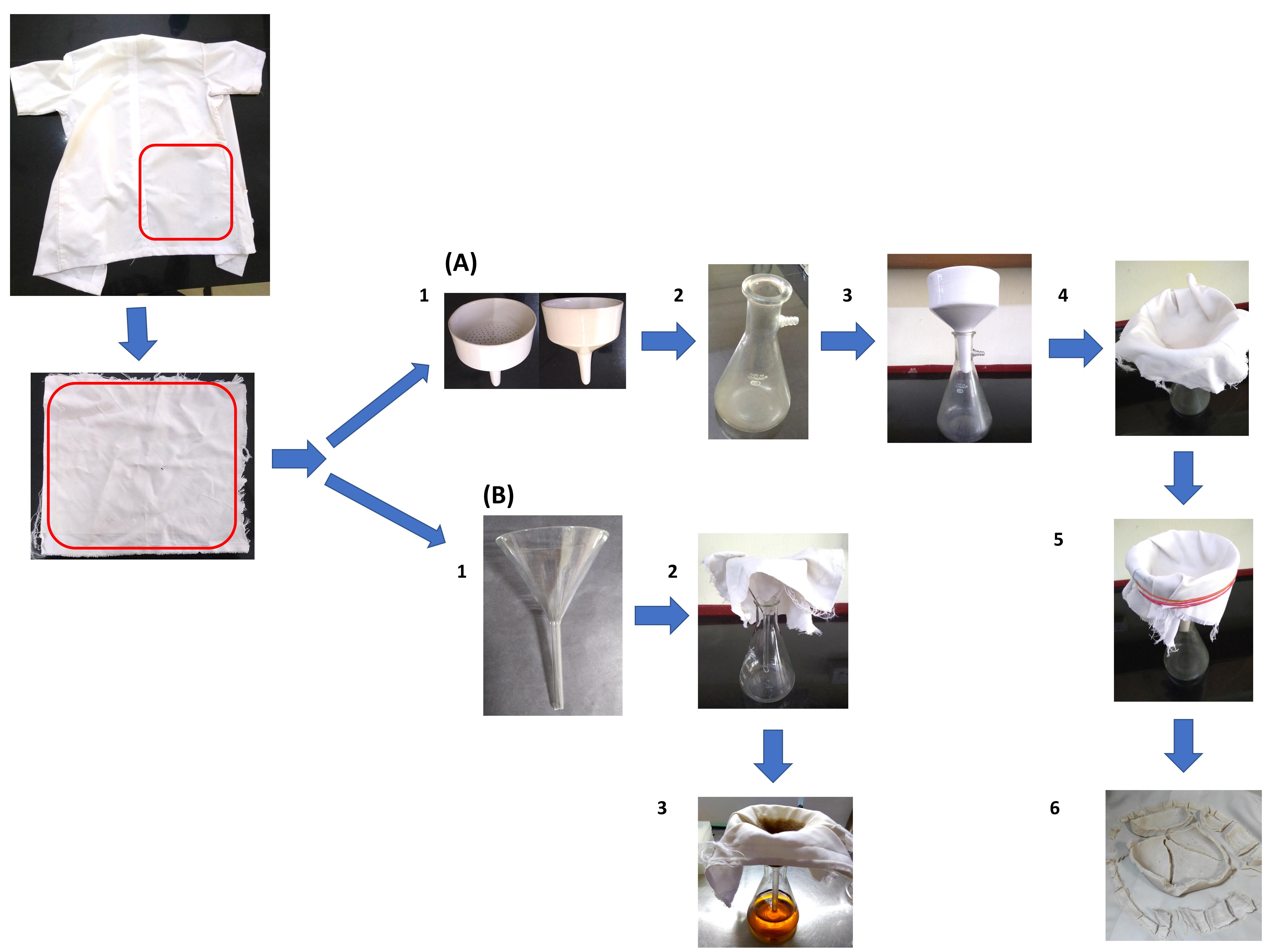
Figure 1. Laboratory coat clothing material (LCCM) based filtration apparatus. (A) Preparation of colloidal chitin (CC). (B) Cell free supernatant (CFS). A piece of LCCM is taken from an old laboratory coat.Video 1. Assembling the LCCM based filtration apparatus and preparation of CCSlowly pour the acidic CC suspension into the Buchner funnel. This solution is still considerably acidic at this stage. Exercise caution while handling.
A clear filtrate will collect in the Buchner flask, and CC is trapped in the LCCM. A vacuum pump may optionally be used to expedite the process.
Decant all of the suspension into the funnel. Allow water to drain from the thick CC paste trapped in the LCCM. This may take 30-45 min without a vacuum pump.
Carefully remove the rubber bands from the filtration apparatus and remove the LCCM from the funnel. Keep the LCCM on a flat surface. Scrape and transfer all of the thick CC paste into a 2 L beaker filled with distilled water. Use a magnetic stirrer and mix this suspension for 15-20 min.
Wash the LCCM in tap water and dry it if you intend to reuse it. The drying step may be avoided, but in our experience, it considerably improves the filtration rate. Reassemble the filtration apparatus as per step 6 and repeat the filtration process (Steps 7-11).
This process must be repeated until the pH of the CC slurry is near neutral. Usually, 3-5 repetitions of the filtration process are required to get near neutral cake. The number of repetitions can be reduced by allowing more time for the water to drain from CC paste (Step 9). Each filtration cycle takes about 2 h to complete.
Collect the CC paste and sterilize it in an autoclave or a pressure cooker at 121°C for 15min. Use steam indicator tape to validate the sterilization process.
Store the sterilized CC at 4°C until use in a tightly closed container. Under this condition, the product can be stored for at least 6 months.
A2. Preparation of CFS
This procedure is best suited for fungi and actinomycetes that exhibit pellet growth.
Sterilize the LCCM and conical flask cap in an autoclave or pressure cooker at 121°C for 15 min. Sterilize a conical flask and a glass funnel in a hot air oven at 170°C for 45 min. Use appropriate indicator tapes/strips to validate the sterilization process.
Assemble the CFS filtration apparatus as depicted in Figure 1B under sterile conditions inside a laminar flow chamber. Use sterile tweezers to push the LCCM into the funnel.
The use of rubber bands for securing the LCCM to the funnel is not required.
A liquid culture of Streptomyces rimosus AFM-1 grown in colloidal chitin mineral salts medium (CCMS) was used in this study. The culture exhibited pellet growth. Pour the contents of the fermented broth into the funnel wrapped in LCCM. Wait for the liquid in the funnel to filter into the flask before topping it up.
Close the glass flask with the cap and store at 4 °C until further use.
Either collect or dispose of the cell mass as per requirement. Decontaminate the cell mass and LCCM before disposal or cleaning, respectively.
Extraction and plate assay of laminarinase from Streptomyces rimosus AFM-1
B1. AS precipitation
Use powdered AS if available, or else grind the granules to a fine consistency using a mortar and pestle. Powdered AS is easy to dissolve in the CFS.
Mixing of AS with CFS should be performed at 4°C to avoid denaturation of proteins. Use crushed ice if cold room facility is not available.
Weigh the required amount of powdered AS. Add small portions to the CFS. Use a magnetic stirrer at low speed for mixing. Ensure that the previously added portion is completely dissolved before adding the next. High mixing speed or large portions of AS cause frothing and concomitant loss of proteins.
Continue stirring the CFS at cold temperature for at least 30 min.
Centrifuge at 4,032 × g for 30 min to pellet the precipitated proteins. The centrifugation parameters must be optimized for different proteins.
Decant the supernatant and dissolve the protein pellet in a small volume of 50 mM potassium phosphate buffer (pH 6.5) or any other buffer system of your choice. The enzyme content of this solution can be directly analyzed by laminarin-infused agarose plate assay.
Similar precipitation experiments can be performed with different concentrations of AS (viz. 30-90% saturation). Such experiments can be used to optimize the AS saturation needed for precipitation while simultaneously reducing the levels of contaminating proteins (Figure 2).
B2. Testing enzyme solutions using laminarin infused agarose plate with OFB staining
Prepare a solution of 0.8% agarose and 0.15% laminarin in 50 mM potassium phosphate buffer (pH 6.5) by melting the solution in a microwave oven and pour into a clean Petri dish. Wait for the gel to solidify.
Using a cork borer, cut 6 mm diameter wells in the agarose gel.
Pour 20-50 µl of laminarinase enzyme or AS precipitated samples into the wells. Use the same volume of either plain buffer or heat-inactivated enzyme solution as a negative control. Refer to the notes section for further instructions.
Place the Petri dish in an incubator set at 37°C. Standardize incubation time and temperature for your application before running the full-scale experiments.
Meanwhile, prepare a 0.15% solution of OFB Tinopal CBS-X in distilled water. Prepare this solution fresh in a container protected from light.
After the incubation time of 4 h, flood the surface of the agarose gel with the OFB solution. Incubate the plate for 15 min in the dark. Discard the OFB solution and destain once with distilled water for 20 min.
Observe the Petri dish under 365 nm UV light (Figure 2A and 2B). An antibiotic zonescale or a calibrated scale ruler is recommended for measuring the zones of hydrolysis.
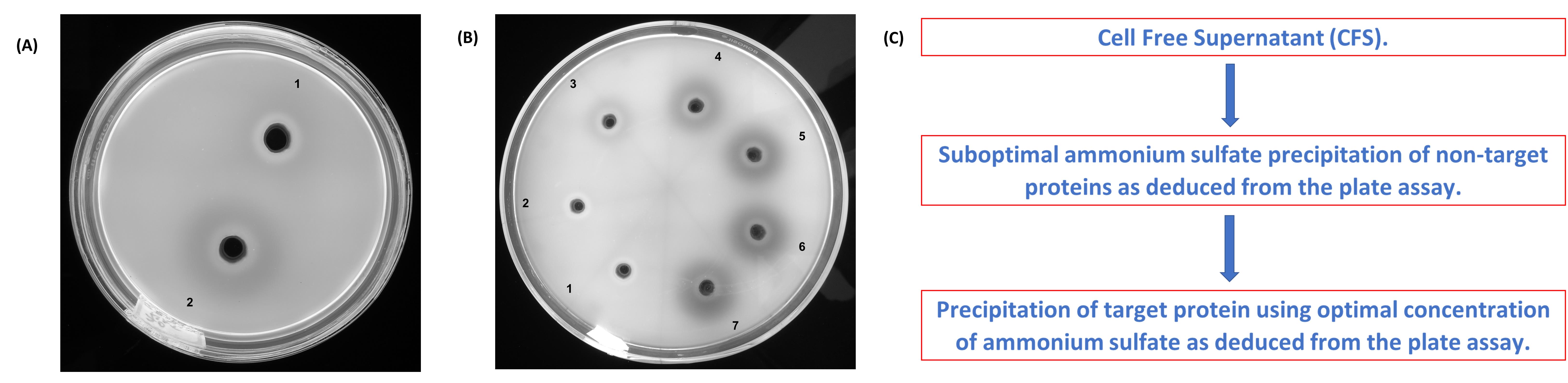
Figure 2. Optimizing the ammonium sulfate (AS) concentration based on laminarin infused agarose plate assay. (A) Heat denatured (1) and native (2) samples of AS precipitated laminarinase. (B) Enzyme precipitates (30-90% AS treatment). 30% (1); 40% (2); 50% (3); 60% (4); 70% (5); 80% (6) and 90% (7). (C) A suggestive precipitation protocol for improved selectivity.
Determination of average Feret’s pore diameter of LCCM (see Video 2)
Place the graticule in the eyepiece of a microscope as per the manufacture’s recommendation.
Place the stage micrometer in the microscope and focus using 4× objective.
Align the markings on micrometer and the graticule. Take a picture (P1). This picture will be used for ‘define scale’ function of ImageJ software.
Remove the stage micrometer and carefully place a piece of LCCM without disturbing the focus settings.
Nudge the LCCM to bring it into sharp focus. Take a picture with the LCCM in focus (P2). This picture will be used to measure the average Feret’s pore diameter in the LCCM.
Download the latest Java runtime environment (JRE) and install.
Download Fiji software and unpack the zip file (Rueden et al., 2017). This software is provided as a portable application and hence installing is not necessary.
Double click on the ImageJ application file located inside the unpacked file folder.
The ‘Analyze’ menu is important for this work. Most of the functions used here are under this menu (viz. ‘Set scale’ and ‘Measure’).
Open P1 in ImageJ. Select straight line tool. Draw a line from the left-hand end of the micrometer markings to where the lines on the stage micrometer and graticule align with each other (Figure 3A).
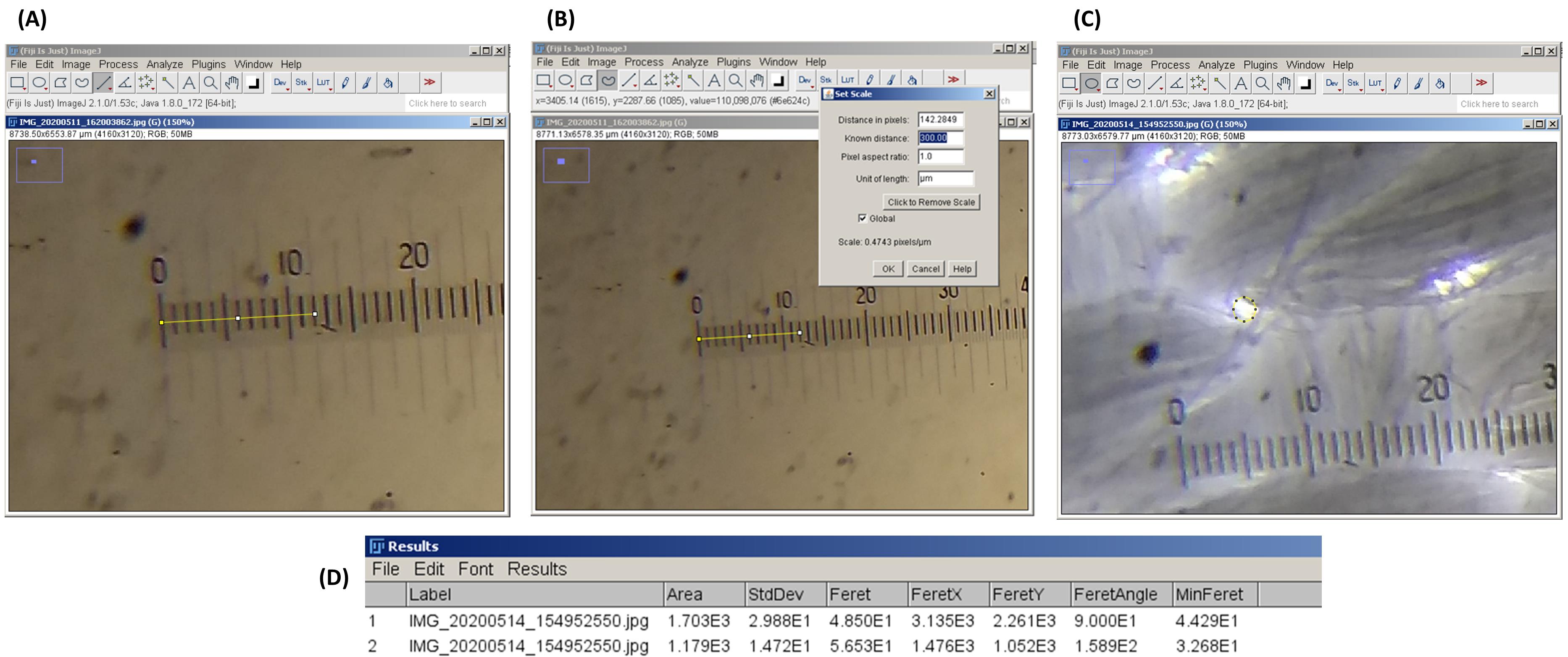
Figure 3. Use of ImageJ for the determination of Feret’s average pore diameter. (A) Drawing a straight line in ImageJ. Stage micrometer and eyepiece graticule are aligned with each other. (B) Setting the measurement scale (‘set scale’ function). Count the number of markings on the stage micrometer that align with the markings on the eyepiece graticule. (C) Selecting a pore in LCCM using the oval selection tool. (D) The ‘Results’ window of ImageJ. Note the dimensional parameters of the selected area.Note the number of markings in the stage micrometer that align with the graticule markings. For example, in Figure 3A, it is at the third marking of stage micrometer, which is equivalent to 300 μm.
Open the ‘set scale’ option and input 300 in the ‘known distance’ menu. Type ‘um’ in the ‘unit of length’ menu. ImageJ will recognize this as μm. Click the ‘global’ check box and press OK (Figure 3B).
ImageJ is now calibrated to perform measurement operations. Open P2 without exiting the software.
Select the oval or freehand selection tool. Select a pore in P2 (Figure 3C).
Click on ‘measure’ option. A new ‘Results’ window will open, which includes all the dimensional parameters of the selected area in P2 (Figure 3D).
Note down the Feret’s diameter.
Select another pore and repeat Steps 14-16. Analyze as many pores in P2 per requirement and tabulate the results. Calculate the average value. This will be the average Feret’s pore diameter of LCCM.
Video 2. Determination of average Feret’s pore diameter using ImageJ tool
Data analysis
Testing enzyme solutions using laminarin infused agarose plate with OFB staining
The zones of hydrolysis can be measured using scale ruler or antibiotic zonescale.
Duplicate samples can be included to enhance precision.
Calculate the average and, if required, the standard deviation of zone diameters.
No special mathematical/statistical tools are needed for this task.
Refer to Koteshwara et al. (2021) (supplementary material) for more information.
Determination of average Feret’s pore diameter of LCCM
Use at least three different LCCM pictures for pore size determination.
Tabulate the Feret’s diameter values from the ImageJ software.
Find the average of Feret’s diameter of pores. A graph denoting the distribution of pore sizes can also be prepared.
Refer to Koteshwara et al. (2021) (supplementary material) for more information and graph.
No special mathematical/statistical tools are needed for this task.
Notes
For the laminarin plate assay, run a few trial experiments if the concentration of reagents or buffer systems are changed from what is described here.
Cost-effective alternatives are available for most of the items listed under reagents and equipment.
The laminarin infused agarose gel method with OFB staining described here can be used to optimize the AS concentration and precipitation time (see Koteshwara et al., 2021) for more information) without dialyzing the sample. Data from the optimization experiment can be used to reduce the amount of contaminating proteins (Figure 2B and 2C).
Buffer system and laminarin concentration can be changed based on the requirements. Vary the volume of the laminarin-infused agarose gel based on the diameter of the Petri dish used to get the required gel thickness. We recommend adding 25 ml and 65 ml, respectively, for 3.9” and 5.9” diameter Petri dishes.
Use sodium azide to inhibit microbial growth in the laminarin-infused agarose gel (see Recipes).
Boiling enzyme samples at 100°C for 20 min usually denatures most proteins. Run an experiment to confirm inactivation. Denatured enzyme samples can be used as a negative control.
A vacuum pump may be used to speed up CC filtration.
Pressure cookers are most convenient for sterilizing small batches of articles. We have been successfully using a 16 L pressure cooker with liquified petroleum gas (LPG) stove for the past several years.
There may be other methods for the determination of the average Feret’s pore diameter using ImageJ, but the procedure described here is direct and simple.
The methods described here are simple, inexpensive, and easy to perform in most of the research laboratories without a large capacity centrifuge for the preparation of CC and CFS. Dialysis, a time-consuming procedure followed commonly during optimization of AS concentration and precipitation time, can be avoided by using the laminarin plate assay described here.
Recipes
Laminarin infused agarose gel
50 mM potassium phosphate buffer
0.15% laminarin
0.8% agarose
0.05% sodium azide (optional)
pH 6.5
Dissolve 0.294 g of K2HPO4, 0.451 g of KH2PO4, 0.15 g of laminarin, 0.05 g of sodium azide (optional), and 0.80 g of agarose in 100 ml distilled water. Heat to dissolve and pour into Petri dish.
Tinopal CBS-X OFB staining solution
0.15% Tinopal CBS-X 99% pure
Dissolve 0.15 g OFB in 100 ml distilled water.
Chitin acid slurry
10 g shrimp chitin
37%w/w HCl
Slowly mix 10 g chitin in 100 ml of HCl. Proceed as per section A1.
CCMS broth medium
Dissolve 2.803 g K2HPO4, 1.893 g KH2PO4, 1.5 g NaCl, 3 g yeast extract, 0.5 g MgSO4·5H2O, 0.01 g FeSO4·7H2O, 0.001 g ZnSO4, 25 g moist CC in 1,000 ml of distilled water. Adjust to pH 7.0.
Acknowledgments
This work was supported by the Department of Biotechnology, Ministry of Science and Technology, Government of India (project ID: BT/PR10827/AAQ/3/661/2014) and was the subject of previous work by Koteshwara et al. (2021). The author wishes to thank Mr. Gayam Prasanna Kumar Reddy, a doctoral student, for his help in videography.
Competing interests
The author declares no competing interests.
Ethics
No human subjects or animals were used for this research work.
References
- Aktuganov, G., Melentjev, A., Galimzianova, N., Khalikova, E., Korpela, T. and Susi, P. (2008). Wide-range antifungal antagonism of Paenibacillus ehimensis IB-X-b and its dependence on chitinase and β-1,3-glucanase production. Can J Microbiol 54(7): 577-587.
- Beattie, A., Hirst, E. L. and Percival, E. (1961). Studies on the metabolism of the Chrysophyceae. Comparative structural investigations on leucosin (chrysolaminarin) separated from diatoms and laminarin from the brown algae. Biochem J 79: 531-537.
- de la Cruz, J., Pintor-Toro, J. A., Benitez, T., Llobell, A. and Romero, L. C. (1995). A novel endo-β-1,3-glucanase, BGN13.1, involved in the mycoparasitism of Trichoderma harzianum. J Bacteriol 177(23): 6937-6945.
- Duong-Ly, K. C., and Gabelli, S. B. (2014). Salting out of proteins using ammonium sulfate precipitation. (Chapter seven). In: Meth Enzymol. Lorsch, J. (Ed.). 541: 85-94.
- Graiff, A., Ruth, W., Kragl, U. and Karsten, U. J. (2016). Chemical characterization and quantification of the brown algal storage compound laminarin—A new methodological approach. J Appl Phycol 28(1): 533-543.
- Grinter, R. and Lithgow, T. (2020). The crystal structure of the TonB-dependent transporter YncD reveals a positively charged substrate-binding site. Acta Crystallogr D Struct Biol 76(Pt 5): 484-495.
- Gulati, R., Saxena, R. K. and Gupta, R. (1997). A rapid plate assay for screening L-asparaginase producing micro-organisms. Lett Appl Microbiol 24(1): 23-26.
- Hsu, S. C. and Lockwood, J. L. (1975). Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl Microbiol 29(3): 422-426.
- Javaid, A. and Ali, S. (2011). Herbicidal activity of culture filtrates of Trichoderma spp. against two problematic weeds of wheat. Nat Prod Res 25(7): 730-740.
- Koteshwara, A., Philip, N. V., Aranjani, J. M., Hariharapura, R. C. and Volety Mallikarjuna, S. (2021). A set of simple methods for detection and extraction of laminarinase. Sci Rep 11(1): 2489.
- Meddeb-Mouelhi, F., Moisan, J. K. and Beauregard, M. (2014). A comparison of plate assay methods for detecting extracellular cellulase and xylanase activity. Enzyme Microb Technol 66: 16-19.
- Mitsutomi, M., Hata, T. and Kuwahara, T. J. (1995). Purification and characterization of novel chitinases from Streptomyces griseus HUT 6037. J Ferment Bioeng 80(2): 153-158.
- Murthy, N. S. and Bleakley, B. J. (2012). Simplified method of preparing colloidal chitin used for screening of chitinase- producing microorganisms. Internet J Microbiol 10(1): 1-5.
- Nidheesh, T. and Suresh, P. V. (2015). Optimization of conditions for isolation of high quality chitin from shrimp processing raw byproducts using response surface methodology and its characterization. J Food Sci Technol 52(6): 3812-3823.
- Park, S. R., Lim, C. Y., Kim, D. S. and Ko, K. (2015). Optimization of Ammonium Sulfate Concentration for Purification of Colorectal Cancer Vaccine Candidate Recombinant Protein GA733-FcK Isolated from Plants. Front Plant Sci 6: 1040.
- Patil, S. and Chaudhari, B. (2017). A simple, rapid and sensitive plate assay for detection of microbial hyaluronidase activity. J Basic Microbiol 57(4): 358-361.
- Qin, H. M., Miyakawa, T., Inoue, A., Nakamura, A., Nishiyama, R., Ojima, T. and Tanokura, M. (2017). Laminarinase from Flavobacterium sp. reveals the structural basis of thermostability and substrate specificity. Sci Rep 7(1): 11425.
- Rueden, C. T., Schindelin, J., Hiner, M. C., DeZonia, B. E., Walter, A. E., Arena, E. T. and Eliceiri, K. W. (2017). ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform 18(1): 529.
- Samad, M. Y. , Razak, C. N., Salleh, A. B., Yunus, W. Z., Ampon, K. and Basri, M. J. (1989). A plate assay for primary screening of lipase activity. J Microbiol Methods 9(1): 51-56.
- Sandhya, C., Adapa, L. K., Nampoothiri, K. M., Binod, P., Szakacs, G. and Pandey, A. (2004). Extracellular chitinase production by Trichoderma harzianum in submerged fermentation. J Basic Microbiol 44(1): 49-58.
- Sarkar, S., Pramanik, A., Mitra, A. and Mukherjee, J. (2010). Bioprocessing data for the production of marine enzymes. Mar Drugs 8(4): 1323-1372.
- Sawant, S. S., Salunke, B. K. and Kim, B. S. (2015). A rapid, sensitive, simple plate assay for detection of microbial alginate lyase activity. Enzyme Microb Technol 77: 8-13.
- Scott, J. H. and Schekman, R. J. (1980). Lyticase: endoglucanase and protease activities that act together in yeast cell lysis. J Bacteriol 142(2): 414-423.
- Teather, R. M. and Wood, P. J. (1982). Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43(4): 777-780.
- Watanabe, T., Yahata, N., Nakamura, Y., Muramoto, Y., Suzuki, K., Kamimiya, S., Tanaka, H. and Watanabe, T. (1989). Expression in Escherichia coli of the Bacillus circulans wl-12 structural gene for β-1,3-glucanase a. Agric Biol Chem 53(7): 1759-1767.
- Weiss, M. S., Abele, U., Weckesser, J., Welte, W., Schiltz, E. and Schulz, G. E. (1991). Molecular architecture and electrostatic properties of a bacterial porin. Science 254(5038): 1627-1630.
- Wingfield P. (2001). Protein precipitation using ammonium sulfate. Curr Protoc Protein Sci Appendix 3, Appendix–3F.
- Wood, P. J., Erfle, J. D. and Teather, R. M. (1988). Use of complex formation between congo red and polysaccharides in detection and assay of polysaccharide hydrolases. Meth Enzymol 160(C): 59-74.
- Wu, Q., Dou, X., Wang, Q., Guan, Z., Cai, Y. and Liao, X. (2018). Isolation of β-1,3-glucanase-producing microorganisms from Poria cocos cultivation soil via molecular biology. Molecules 23(7).
- Yang, J., Xu, Y., Miyakawa, T., Long, L. and Tanokura, M. (2020). Molecular basis for substrate recognition and catalysis by a marine bacterial laminarinase. Appl Environ Microbiol 86(23).
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Koteshwara, A. (2021). Simple Methods for the Preparation of Colloidal Chitin, Cell Free Supernatant and Estimation of Laminarinase. Bio-protocol 11(19): e4176. DOI: 10.21769/BioProtoc.4176.
Category
Microbiology > Microbial biochemistry > Protein
Biochemistry > Carbohydrate > Polysaccharide
Biological Sciences > Microbiology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










