- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Quantitative Analysis of RNA Editing at Specific Sites in Plant Mitochondria or Chloroplasts Using DNA Sequencing
Published: Vol 11, Iss 18, Sep 20, 2021 DOI: 10.21769/BioProtoc.4154 Views: 3291
Reviewed by: Dheeraj Singh RathoreIgnacio LescanoAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Micrografting in Arabidopsis Using a Silicone Chip
Hiroki Tsutsui [...] Michitaka Notaguchi
Jun 20, 2021 7948 Views
A Novel Method to Map Small RNAs with High Resolution
Kun Huang [...] Jeffrey L. Caplan
Aug 20, 2021 4678 Views
Profiling of Single-cell-type-specific MicroRNAs in Arabidopsis Roots by Immunoprecipitation of Root Cell-layer-specific GFP-AGO1
Lusheng Fan [...] Xuemei Chen
Dec 20, 2022 2227 Views
Abstract
Cytidine-to-uridine (C-to-U) RNA editing is one of the most important post-transcriptional RNA processing in plant mitochondria and chloroplasts. Several techniques have been developed to detect the RNA editing efficiency in plant mitochondria and chloroplasts, such as poisoned primer extension (PPE) assays, high-resolution melting (HRM) analysis, and DNA sequencing. Here, we describe a method for the quantitative detection of RNA editing at specific sites by sequencing cDNA from plant leaves to further evaluate the effect of different treatments or plant mutants on the C to U RNA editing in mitochondria and chloroplasts.
Keywords: C to U RNA editingBackground
C to U RNA editing is one of the most important post-transcriptional modifications that occur in the plant mitochondrial or chloroplast genes, which usually changes the first or second positions of nucleic acid triplet codons leading to altered protein sequences and is essential for their normal functions (Takenaka et al., 2013; Yan et al., 2018). The RNA editing and processing in mitochondria or chloroplasts have been reported to function in plant male sterility, seed development, adaptations to the environment, and resistance to pathogens (Hammani et al., 2011; Dahan and Mireau, 2013; Garcia-Andrade et al., 2013; Barkan and Small, 2014; Ren et al., 2020; Yang et al., 2020). Several methods have been established for the detection of RNA editing sites or editing levels, such as poisoned primer extension (PPE) assays, high-resolution melting (HRM) analysis, and DNA sequencing (Roberson et al., 2006; Chateigner-Boutin et al., 2007; Hayes and Hanson, 2007). However, the PPE assays usually require radiolabeled oligonucleotides (Hayes and Hanson, 2007). It is hard to distinguish the editing levels at two very close editing sites using HRM assays (Chateigner-Boutin et al., 2007). Currently, DNA sequencing has been an accurate, economic, and widely used method for the RNA editing assays (Bentolila et al., 2012; Brehme et al., 2015; Yang et al., 2017; He et al., 2018). Here, we describe a method for the RNA editing detection by DNA sequencing of cDNA to further evaluate the effects of different treatments or plant mutants on the C to U RNA editing in mitochondrial and chloroplast transcripts (Yang et al., 2020).
Materials and Reagents
Pipette tips and tubes (Axygen, different sizes, and types)
Nicotiana benthamiana leaves
TRIzolTM Reagent (Invitrogen, catalog number: 15596026)
PrimeScript RT Reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa, catalog number: DRR047A)
FastPfu DNA Polymerase (TransGen Biotech, catalog number: AP221-01)
Liquid nitrogen
Gel loading dye
TAE buffer (Dunker et al., 2021)
Agarose (Invitrogen, catalog number: 75510019)
Marker III (TIANGEN Biotech, catalog number: MD103)
Equipment
Thermal Cycler (Bio-Rad, model: S1000)
Refrigerated Centrifuge (Thermo Fisher, model: Legend Micro 17R)
Electrophoresis System (BEIJING LIUYI BIOTECHNOLOGY CO., LTD., DYY-7C)
PIPETMAN Pipettes (Gilson, models: P1000, P100, P20, P2, catalog numbers: F123602, F123615, F123600, F144801)
Software
BioEdit (https://bioedit.software.informer.com/)
Primer Premier 5 (https://primer-premier-5.software.informer.com/)
Procedure
Primer design and template generation
To detect the RNA editing at a specific or several RNA editing sites in mitochondria or chloroplast transcripts, the forward and reverse primers could be designed according to sequences ~100 bp upstream and downstream of the editing sites using Primer 5 or other primer designing software (Note 1), with a recommended primer length of ~20 nt and melting temperature of ~60°C.
Total RNAs from plant tissues are isolated with the TRIzolTM Reagent according to the manufacturer’s instructions and the protocols (MacRae, 2007) (Note 2).
About 800 ng total RNAs were reverse-transcribed into cDNA using a PrimeScript RT Reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa) according to the manufacturer’s instructions with the final volume up to 20 μl (Note 3).
PCR amplification and DNA sequencing of the targeted editing sites
Perform PCR amplification of the fragments that span the targeted RNA editing sites using high-fidelity DNA polymerase.
Set up the PCR, adding the following components into a 0.2 ml PCR tube with the final volume up to 30 μl:
ddH2O 18.4 μl
5× FastPfu Buffer 6 μl
2.5 mM dNTPs 2.4 μl
Forward primer (10 μM) 0.8 μl
Reverse primer (10 μM) 0.8 μl
FastPfu DNA Polymerase (2.5 U/μl) 0.6 μl
Template 1 μl
Run PCR reactions:
1 cycle 95°C, 2 min
34 cycles 95°C, 20 s; 55°C, 20 s; 72°C, 20 s
1 cycle 72°C, 2 min
Perform gel electrophoresis (2% agarose) with half of the PCR products to confirm whether the amplification is specific and correct in size.
Perform DNA sequencing with the other half of the PCR products using the amplification primers by a DNA sequencing service provider (Note 4).
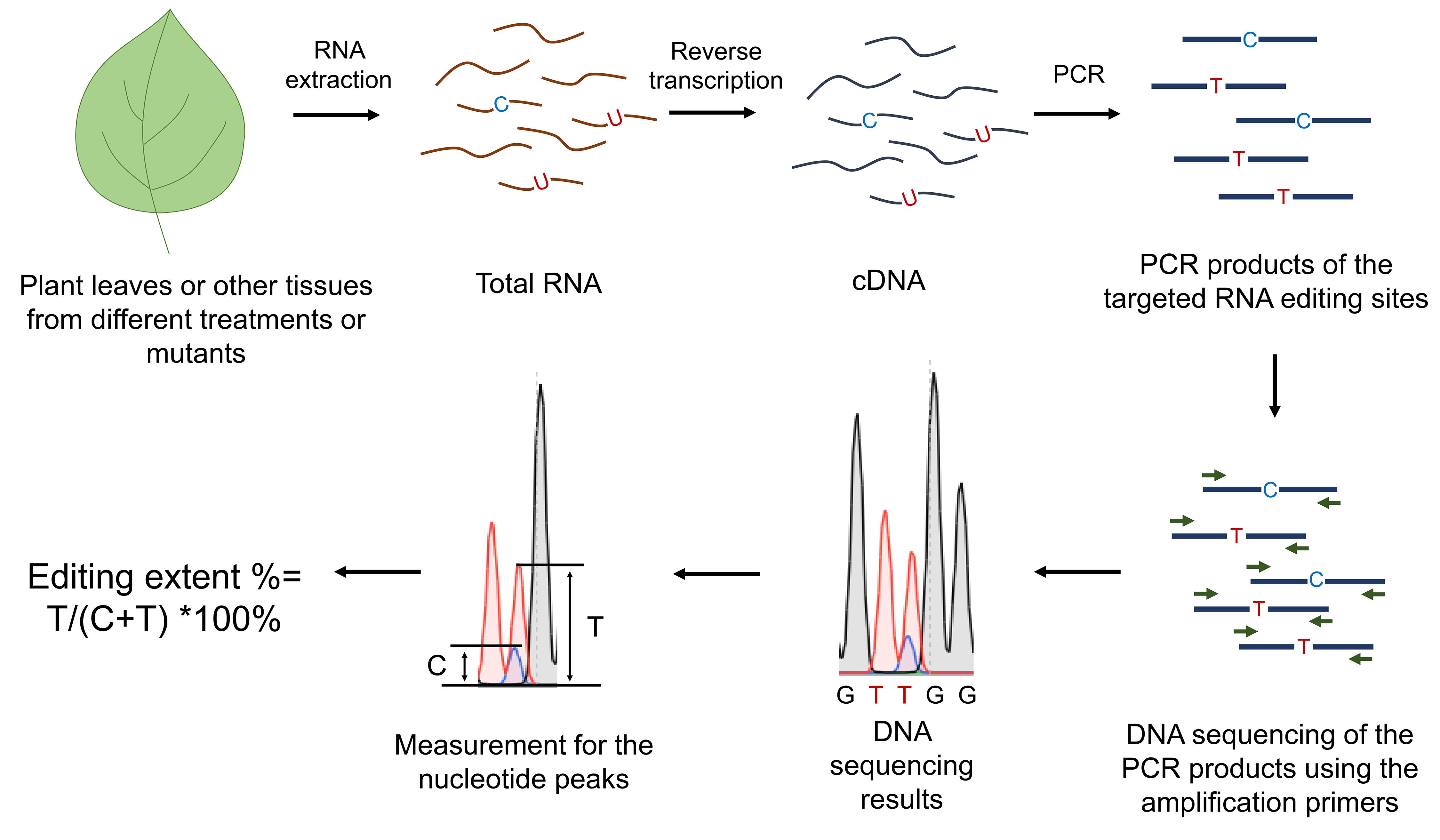
Figure 1. Workflow of the RNA editing analysis of specific sites in mitochondria and chloroplasts
Data analysis
As shown in Figure 1, cDNA sequences were evaluated for their respective C to T differences. The extent of RNA editing was estimated by the relative height of the respective nucleotide peaks in the sequence analysis using the BioEdit software. The editing levels are calculated as editing efficiency %= T/(C+T) ×100%.
Here, we show an example of our previously published work (Yang et al., 2020). We analyzed the C to U RNA editing extent of several mitochondrial genes in NbMORF8-silenced N. benthamiana leaves using GFP silenced leaves as the control. We used one site as an example to indicate the quantitative measurement of the peaks using BioEdit (Figure 2).
Open the sequencing files by BioEdit software.
Put the black cross at the top of targeted nucleotide peaks (nucleotide T and C, respectively) and read the second number, which shows the peak height.
Calculate the editing extent as efficiency %= T/(C+T) ×100%
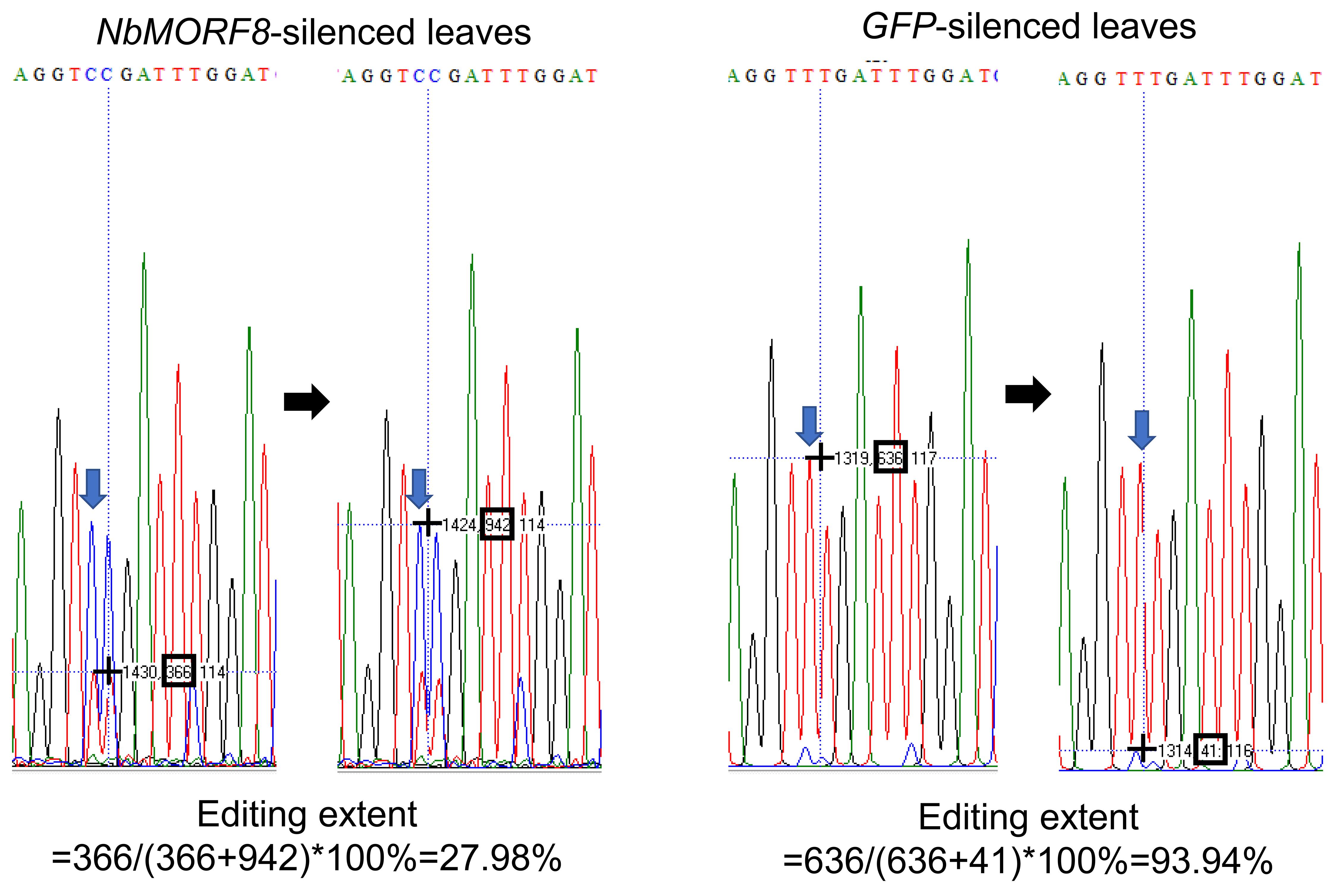
Figure 2. Analysis of RNA editing extent by Bioedit. The blue arrows mark the targeted nucleotide peaks, and the black hollow boxes mark the reads of the peak heights.
Notes
The size of the PCR fragments is recommended in the range of 150-300 bp.
The quality of the total RNA is crucial. Therefore, it is recommended to use the best RNA isolation method optimized for different plant species.
Since genomic DNA sequences represent an unedited version of the targeted transcripts, it is important to erase the genomic DNA to avoid its interference with the RNA editing analyses.
The PCR products are recommended to be sequenced in both forward and reverse directions since the sequencing quality from one side sometimes is not sufficient for further analysis. In our case, the DNA sequencing was conducted by a sequencing company using Applied Biosystems 3730XL.
Acknowledgments
This work was supported by China Agriculture Research System (CARS-09), National Natural Science Foundation of China (31125022 and 31930094), and the Program of Introducing Talents of Innovative Discipline to Universities (project 111) from the State of Administration for Foreign Experts Affairs, P. R. China (B18042). This protocol is adapted from Yang et al. (2020) that we previously published in Plant Physiology.
Competing interests
The authors declare no conflict of interests.
References
- Barkan, A. and Small, I. (2014). Pentatricopeptide repeat proteins in plants. Annu Rev Plant Biol 65: 415-442.
- Bentolila, S., Heller, W. P., Sun, T., Babina, A. M., Friso, G., van Wijk, K. J. and Hanson, M. R. (2012). RIP1, a member of an Arabidopsis protein family, interacts with the protein RARE1 and broadly affects RNA editing. Proc Natl Acad Sci U S A 109(22): E1453-1461.
- Brehme, N., Bayer-Csaszar, E., Glass, F. and Takenaka, M. (2015). The DYW Subgroup PPR Protein MEF35 Targets RNA Editing Sites in the Mitochondrial rpl16, nad4 and cob mRNAs in Arabidopsis thaliana. PLoS One 10(10): e0140680.
- Chateigner-Boutin, A. L. and Small, I. (2007). A rapid high-throughput method for the detection and quantification of RNA editing based on high-resolution melting of amplicons. Nucleic Acids Res 35(17): e114.
- Dahan, J. and Mireau, H. (2013). The Rf and Rf-like PPR in higher plants, a fast-evolving subclass of PPR genes. RNA Biol 10(9): 1469-1476.
- Dunker, F., Lederer, B. and Weiberg, A. (2021). Plant ARGONAUTE Protein Immunopurification for Pathogen Cross Kingdom Small RNA Analysis. Bio Protoc 11(3): e3911.
- Garcia-Andrade, J., Ramirez, V., Lopez, A. and Vera, P. (2013). Mediated plastid RNA editing in plant immunity. PLoS Pathog 9(10): e1003713.
- Hammani, K., des Francs-Small, C. C., Takenaka, M., Tanz, S. K., Okuda, K., Shikanai, T., Brennicke, A. and Small, I. (2011). The pentatricopeptide repeat protein OTP87 is essential for RNA editing of nad7 and atp1 transcripts in Arabidopsis mitochondria. J Biol Chem 286(24): 21361-21371.
- Hayes, M. L. and Hanson, M. R. (2007). Assay of editing of exogenous RNAs in chloroplast extracts of Arabidopsis, maize, pea, and tobacco. Methods Enzymol 424: 459-482.
- He, P., Xiao, G., Liu, H., Zhang, L., Zhao, L., Tang, M., Huang, S., An, Y. and Yu, J. (2018). Two pivotal RNA editing sites in the mitochondrial atp1mRNA are required for ATP synthase to produce sufficient ATP for cotton fiber cell elongation. New Phytol 218(1): 167-182.
- MacRae, E. (2007). Extraction of plant RNA. Methods Mol Biol 353: 15-24.
- Ren, R. C., Wang, L. L., Zhang, L., Zhao, Y. J., Wu, J. W., Wei, Y. M., Zhang, X. S. and Zhao, X. Y. (2020). DEK43 is a P-type pentatricopeptide repeat (PPR) protein responsible for the Cis-splicing of nad4 in maize mitochondria. J Integr Plant Biol 62(3): 299-313.
- Roberson, L. M. and Rosenthal, J. J. (2006). An accurate fluorescent assay for quantifying the extent of RNA editing. RNA 12(10): 1907-1912.
- Takenaka, M., Zehrmann, A., Verbitskiy, D., Hartel, B. and Brennicke, A. (2013). RNA editing in plants and its evolution. Annu Rev Genet 47: 335-352.
- Yan, J., Zhang, Q. and Yin, P. (2018). RNA editing machinery in plant organelles. Sci China Life Sci 61(2): 162-169.
- Yang, Y., Fan, G., Zhao, Y., Wen, Q., Wu, P., Meng, Y. and Shan, W. (2020). Cytidine-to-Uridine RNA Editing Factor NbMORF8 Negatively Regulates Plant Immunity to Phytophthora Pathogens. Plant Physiol 184(4): 2182-2198.
- Yang, Y., Zhu, G., Li, R., Yan, S., Fu, D., Zhu, B., Tian, H., Luo, Y. and Zhu, H. (2017). The RNA Editing Factor SlORRM4 Is Required for Normal Fruit Ripening in Tomato. Plant Physiol 175(4): 1690-1702.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Yang, Y. and Shan, W. (2021). Quantitative Analysis of RNA Editing at Specific Sites in Plant Mitochondria or Chloroplasts Using DNA Sequencing. Bio-protocol 11(18): e4154. DOI: 10.21769/BioProtoc.4154.
- Yang, Y., Fan, G., Zhao, Y., Wen, Q., Wu, P., Meng, Y. and Shan, W. (2020). Cytidine-to-Uridine RNA Editing Factor NbMORF8 Negatively Regulates Plant Immunity to Phytophthora Pathogens. Plant Physiol 184(4): 2182-2198.
Category
Plant Science > Plant molecular biology > RNA > RNA detection
Molecular Biology > RNA > RNA detection
Developmental Biology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link