- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Simple Spatial-independent Associative and Reversal Learning Task in Mice
(*contributed equally to this work) Published: Vol 11, Iss 15, Aug 5, 2021 DOI: 10.21769/BioProtoc.4108 Views: 3687
Reviewed by: Arnau Busquets-GarciaZheng Zachory Wei

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
In situ Microinflammation Detection Using Gold Nanoclusters and a Tissue-clearing Method
Fayrouz Naim [...] Masaaki Murakami
Apr 5, 2023 2666 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2565 Views
A One-Step Mouse Model of Parkinson’s Disease Combining rAAV-α-Synuclein and Preformed Fibrils of α-Synuclein
Santhosh Kumar Subramanya [...] Poonam Thakur
Dec 5, 2025 1633 Views
Abstract
The ability to adapt one's behavior in response to changing circumstances, or cognitive flexibility, is often altered in neuropsychiatric and neurodevelopmental conditions. In rodents, cognitive flexibility is frequently assessed using associative learning paradigms with a reversal component. The majority of existing protocols rely on unrestrictive exploration with no discouragement of wrong responses and are often influenced by spatial cues, at least during the test's learning phase. Here, we present a rewarded contingency discrimination learning test that minimizes the task's spatial component and contains an element that actively discourages pure exploratory responses. The method described herein is a manual version that can be performed using home-made equipment, but the test setup is amenable to automatization and can be adapted to address more complex cognitive demands, including conditional associative learning, attentional set formation, and attention shifting.
Keywords: Associative learningBackground
Contingency reversal learning tasks, where an animal is trained to associate a cue with a reward or a punishment that later is reversed, are frequently used to study neural mechanisms of cognitive flexibility (Izquierdo et al., 2017). In mice, discrimination and reversal learning can be challenging to assess, partly because of two salient and contrasting aspects of species-specific mouse behavior: novelty-induced anxiety (Crusio, 2001) and the tendency to follow incidentally learned spatial cues (Hebert et al., 2017). The natural defensive behavior of mice limits the use of tests based on negative reinforcement, as they reduce exploration and almost invariably result in the animal not performing any response at all. For example, the addition of punishments to incorrect responses appears to worsen reversal learning proficiency as assessed in a touchscreen paradigm (Jager et al., 2020). On top of this, there are ethical concerns about using negative reinforcements in animal experiments. The use of only positive reinforcers, such as food rewards, also has its drawbacks since the motivation to respond to a reward-associated stimulus may change over time along with satiety (Goltstein et al., 2018).
Furthermore, rodents have a natural tendency to alternate between responses (Lalonde, 2002; Deacon and Rawlins, 2006), and in particular, young individuals tend to explore the consequences of alternative non-rewarded responses provided that these do not trigger an openly aversive effect (Walker et al., 1955; Mechan et al., 2009; Sanderson and Bannerman, 2012). In most operant tasks assessing discrimination learning in mice, wrong responses are simply not rewarded and do not require a corrective action by the mouse, which has to “understand” that the given response was wrong by the absence of a consequence. Time-outs appear insufficient to discourage animals from attempting alternative responses, and these alternative attempts may confound the animals’ cognitive ability with its exploratory attitude (Luo et al., 2020). The introduction of an operant response to re-start a trial is a partial remedy to this drawback; however, the presence of a negative, but not aversive, cue associated with a wrong response is the best way to achieve the highest possible proficiencies in these cognitive tasks.
Reversal learning tasks designed for rodents are often conducted in mazes or operant conditioning chambers, where the rewarded (S+) and non-rewarded (S-) stimuli are set at a physical distance sufficient to be discriminated based on their position. When discrimination between the stimuli is not based on location (e.g., shape, odor, or texture discrimination), the incidental learning of their positions in space generates interference with spatial rules, at least during the test’s procedural learning steps. This phenomenon is known as overshadowing and occurs when a second salient cue, such as a spatial one, is in conflict with the designed S+ stimuli (Sánchez-Moreno et al., 1999). Indeed, it has long been established that rats follow spatial cues as a first strategy to retrieve rewards in a maze (Packard and McGaugh, 1996), and some individuals tend to follow spatial strategies even after having been trained in a non-spatial cue-response task in a water maze (Packard and McGaugh, 1992). Similarly, when initially trained to discriminate odors in the “classical” two-chamber setting of the attentional set-shifting task (Bissonette and Powell, 2012), rodents invariably start following spatial cues to figure out where to find a reward (Tait et al., 2018). Suppression of this spatial bias interfering with the conditioning stimulus may require additional days of training and may – paradoxically – result in worse performance of animals that usually rely more on hippocampal-dependent spatial information: an ecologically more relevant feature when seeking for a reward-related contingency in rodents (Devan and White, 1999; Chang and Gold, 2003). The interference of incidentally learned spatial information can be so relevant that it can result in paradoxically enhanced discrimination learning in hippocampal lesioned mice (Sanderson et al., 2012).
Here, we present a protocol to assess discrimination learning and contingency reversal in a simple home-made setup, under conditions that improve on both the previously described limitations of most current discrimination learning devices. The present protocol minimizes the interference of non-relevant spatial information by concentrating the S+ and S- stimuli on a single, small pedestal placed in a compartment of the arena that animals will recognize as a single “reward zone.” This is a significant difference compared to the arenas used for the classical attentional set-shifting tasks, where the S+ and S- are presented in two separate adjacent compartments (e.g., Colacicco et al., 2002). In addition, we apply a negative response signal by introducing a sliding lid moving inside the test box upon attempts to reach the non-rewarded vial. This step is equivalent to closing the reward receptacle, an operation that can be performed on some other operant tasks (e.g., Krackow et al., 2010). Furthermore, it also acts as a “trial-ended” signal and solicits a withdrawal response in the mouse to the waiting zone, thus prompting a new trial.
Materials and Reagents
15-ml Falcon tubes with lids (BD Biosciences, catalog number: 352096)
Plastic (1.5 mm) and Plexiglas (4 mm) panels (Jumbo-Markt AG, Switzerland)
Sandpaper (Jumbo-Markt AG, Switzerland)
Teflon tape (Jumbo-Markt AG, Switzerland)
Wood block (Jumbo-Markt AG, Switzerland)
Iron plate (0.5 mm) (Jumbo-Markt AG, Switzerland)
Neodymium disc magnets Ø 2 mm, height 2 mm (Jumbo-Markt AG, Switzerland)
Condensed milk cream (9% milk fat, 57% sugar, 8% milk proteins) (Migros, Zürich, Switzerland)
Felt-tip pen (Sharpie Fine, Atlanta, GA, USA)
Video camera (Ikegami ICD47E, 41460 Neuss, Germany)
Monitor (Samsung, model: 710 WP S)
Equipment
Test arena
The test takes place in an arena made with a plastic crate, such as a 20-L EURO Rako box (https://www.utzgroup.ch/stacking-container-rako-400-300-220-mm-161559/). The inner dimensions are 37 × 27 cm, 21.5 cm height. The box is divided into two halves by inserting a 0.4 × 27 cm Plexiglas wall, in which a 5 × 5 cm slot has been cut. One half is the waiting zone, and the other is the reward zone. The opening between the zones can be closed by an opaque polypropylene sliding guillotine door (Figure 1).

Figure 1. Top view of the arenaOn one of the box's short sides, two 52 × 2 mm slots are cut, 5 mm apart from the midline of the short wall, and 35 mm above the floor. In each slot, a plastic L-shaped pad, 15 × 5 cm and 1.5 mm thick, is inserted and used as a sliding lid to cover the vials that will contain the rewards (Figure 2). The last 20 mm of each pad's long side are folded upward to prevent their total extraction from the slots and to gently push away the mouse after the choice has been made.

Figure 2. Reward pedestal with sliding lids, top-front viewBetween the slots with the sliding lids, there is a pedestal carrying the stimuli (S+: reward; S-: no reward). The rewards are placed in two 15-ml Falcon tube lids positioned on the pedestal (see Figure 3). This pedestal is made of a small woodblock, 10 × 4 cm and 15 mm thick, divided into two equal halves by a transparent Plexiglas bar, 22 × 5 cm and 5 mm thick. Each half of the pedestal is lined with a pattern differing in color and texture and carries at its center, under the lining, a thin iron plate. The linings are made of white Teflon tape on one side and brown sandpaper on the other. Alternative linings, such as Velcro tape and PVC from floor tiles, can be used, but their salience equivalence should be checked in advance.
Materials the same as the lining are used to externally line the two lids of 15-ml Falcon tubes used as reward receptacles. A 2-mm magnet is glued under each lined vial to secure it in place on the pedestal.
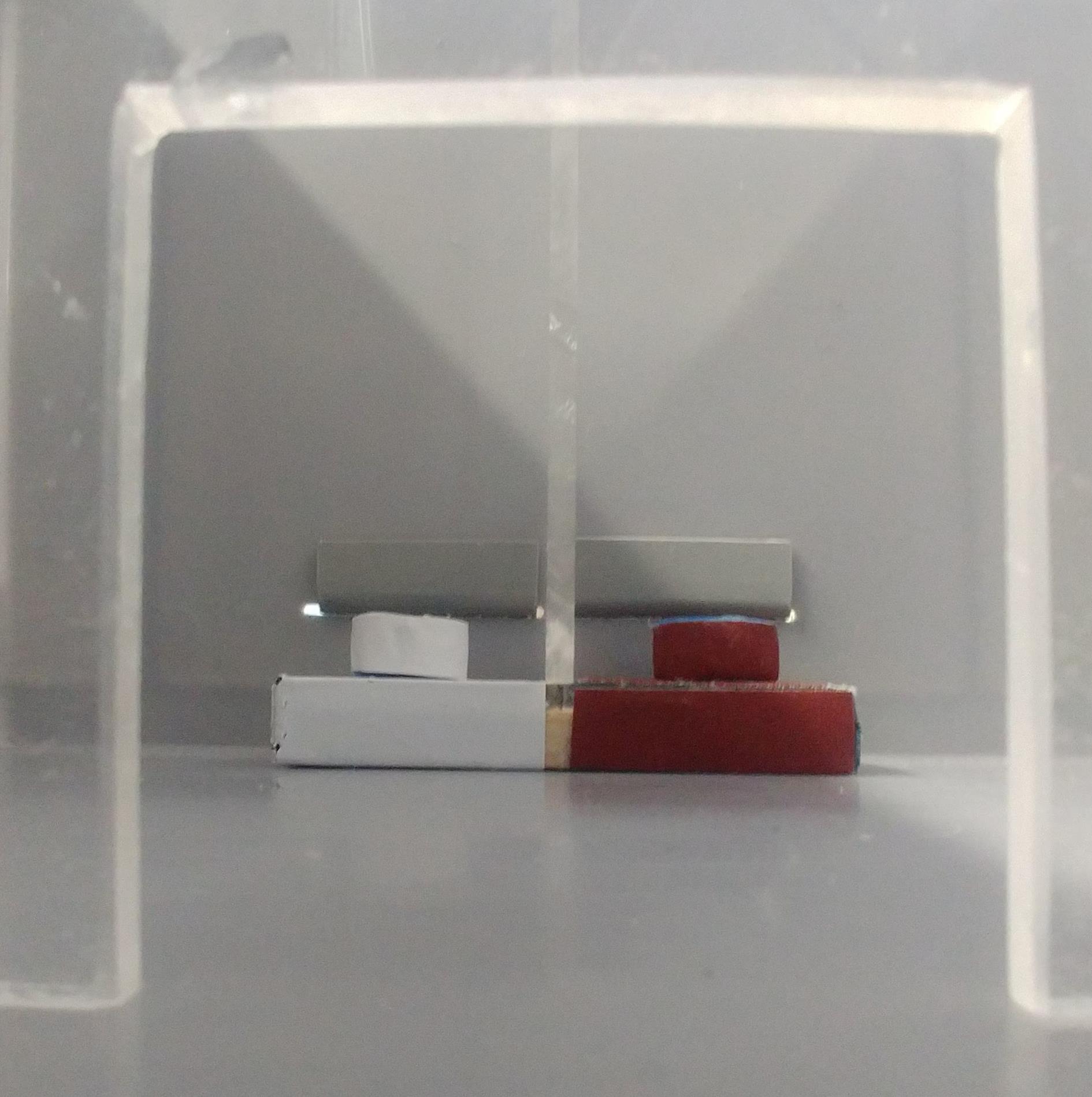
Figure 3. Reward pedestal from the frontDuring training and testing, the arena is placed on a table in a room with indirect illumination at 30-50 lux intensity. The experimenter should avoid bending over the arena while the mice are in the reward zone. Mice are observed via a monitor connected to a video camera.
Video camera and monitor
We used an Ikegami IR ICD47E analogic camera connected to a video monitor via a BMC cable. However, for the sake of monitoring the mice during the test, virtually any camera connected to a personal computer would work.
Procedure
Habituation and procedural learning
Before starting the training sessions, animals must be familiarized with the reinforcers, the experimenter, and the testing arena. Before learning any reward contingency rule, mice have to learn how to control events taking place in the arena.
Habituation to the reward: Three days before starting the testing (Day -3), place 200 μl condensed milk in the vials (lids of 15-ml Falcon tubes, without any liner) and set two of these vials overnight in each cage housing the mice to be tested. Undiluted condensed milk is sticky, and empty vials witness that tasting has occurred.
Habituation to the setup: The following day (Day -2), bring the mice to the testing room and release them in the waiting zone of the testing arena, in the same groups as they are housed. When introduced to a novel environment, rodents feel safer in the presence of their cage-mates, because companionship provides protection from potential environmental threats (Kikusui et al., 2006). On the floor of the reward zone, scatter four vials baited with 100 μl condensed milk diluted 1:1 with water. Allow the mice to roam freely and toggle between the waiting and the reward zone, occasionally activating the door and the sliding lids that will cover the testing vials. Monitor mice behavior via the camera. The session lasts at least 5 min but continues until all the mice have tasted the reward. Recollect the mice and identify them individually with marks on the tails using a non-toxic felt-tip pen. Bring the mice back to their usual cage and, in the evening, re-set the two vials with 200 μl condensed milk in each cage. Weigh the mice and reduce chow food to 1 g per mouse for the night.
Procedural learning: The following day (Day -1), release the mice individually into the waiting zone with the door closed. The pedestal should already be in place in the reward zone, covered with black adhesive tape and carrying two neutral vials (no liner), both baited with 15 μl diluted condensed milk. Open the door and confine the mouse to the reward zone until it consumes the milk in one of the two vials. Monitor mouse behavior via the camera and close the other vial with the sliding lid when the mouse starts licking the milk in the chosen vial. Once the milk is consumed, cover the empty vial, and re-open the gate between the reward and waiting zone. If the mouse does not re-enter the waiting zone spontaneously, gently push it back using a “shovel” identical to the one used as a sliding lid: mice will readily learn to respond to the presentation of this object by retiring to the waiting zone. Close the gate, withdraw the pedestal from the arena, and re-bait the visited vial using a Gilson pipetman; then, re-place the pedestal in the arena and open the gate again. Mice have to learn to toggle between the waiting and reward zone to seek and consume the reward. When a mouse has retrieved at least 4 rewards, and after at least 5 min have passed, withdraw the mouse using a cardboard tunnel (avoid tail handling), refresh the tail mark, and move the mouse back to the home cage. If the mouse has not retrieved 4 rewards after 5 min, allow it to stay longer until 4 rewards have been retrieved, but abort the training after 20 min if the mouse still has not consumed the rewards. When all mice in a batch have been trained, transfer to the vivarium, weigh each mouse, and give food ad libitum. Again, if the animals have not lost more than 10% of their initial body weight, restrict food to 1 g chow/mouse overnight. A second procedural training day (Day 0) may be necessary if more than 2 mice per group did not consume four baits within 20 min.
Learning of the first rule-reward contingency: Day 1 to the day of criterion attainment
Table 1. Example of the daily spreadsheet
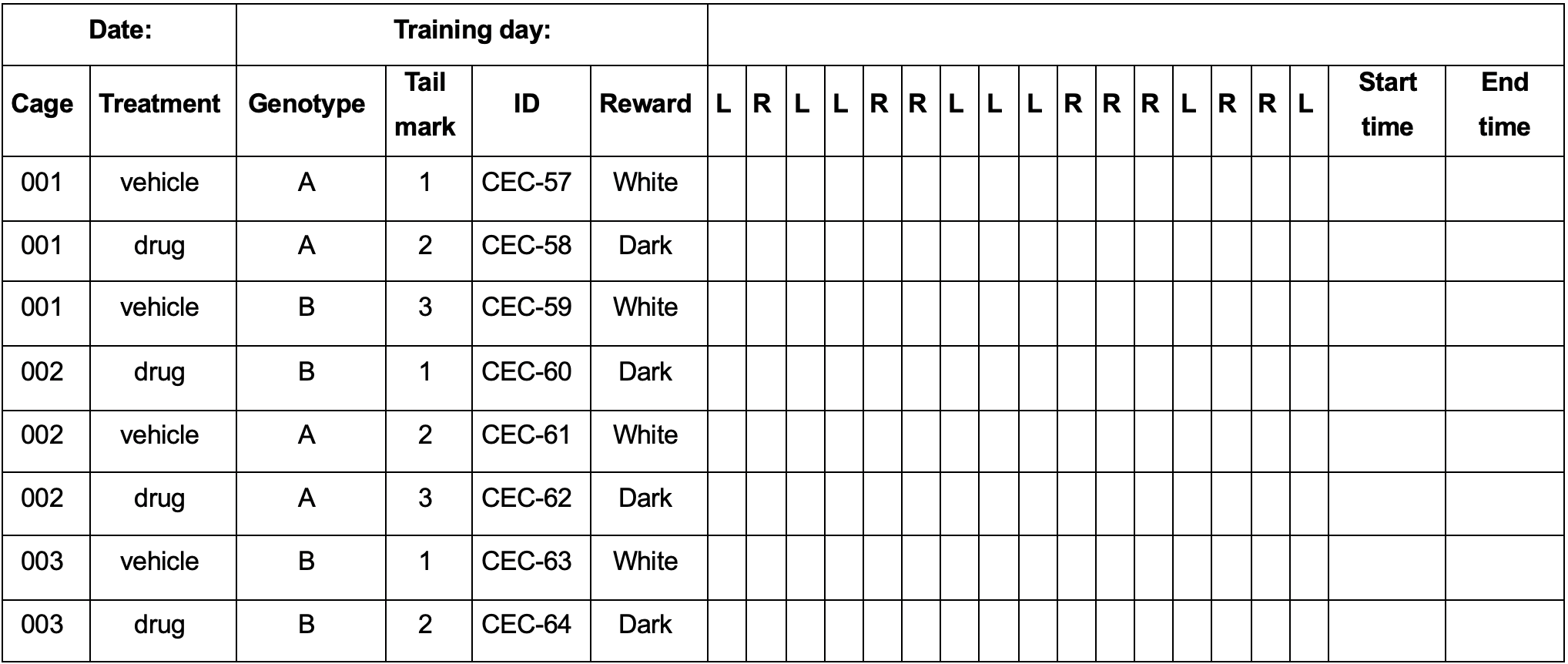
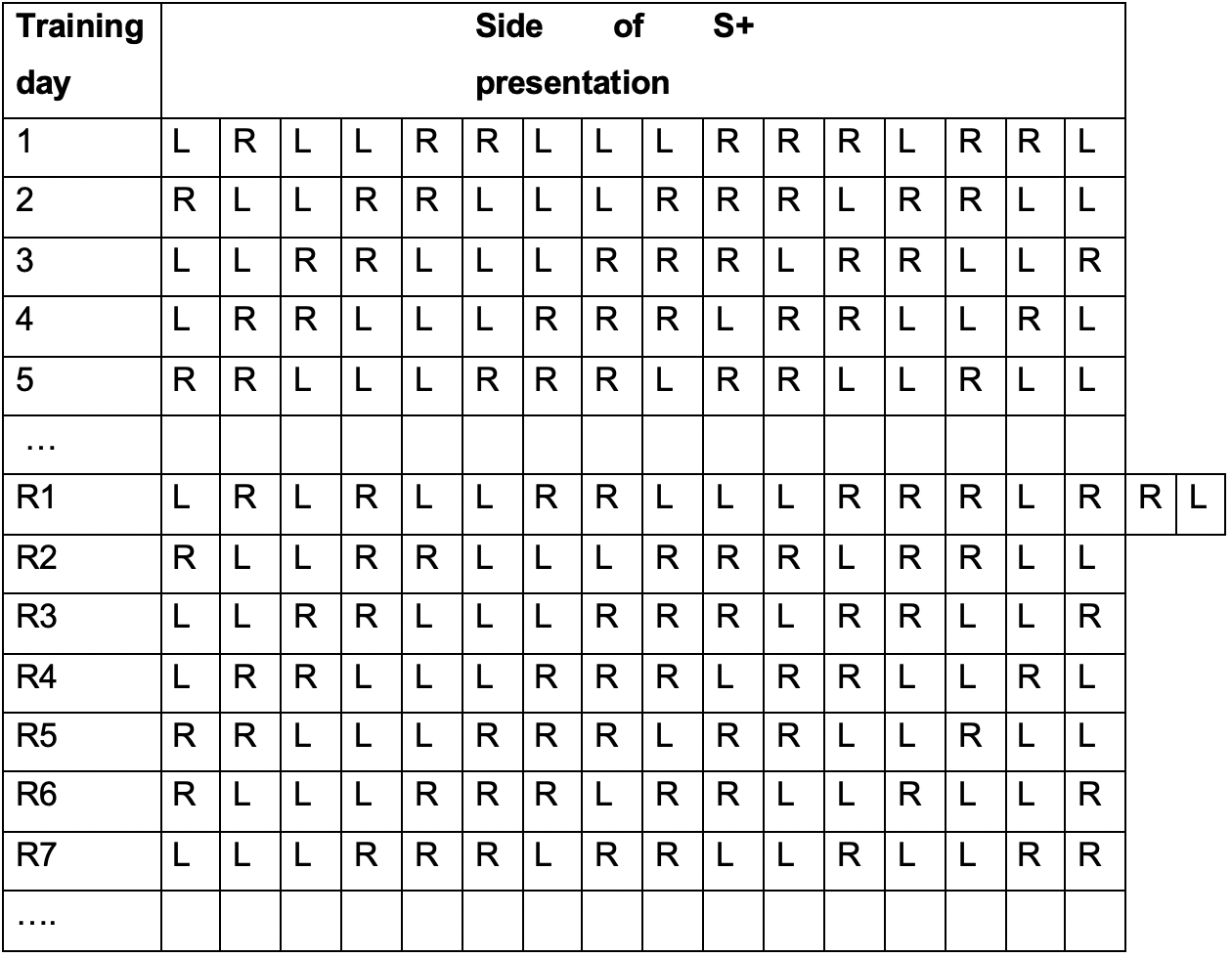
Bring the mice to the test room one hour before starting the training session. Switch on the indirect lights and camera. For reward contingency rule learning, the vials containing the baits and the pedestal should now be lined with patterns: one with white tape and the other with brown sandpaper. Balance the reward contingency among mice, such that an equal number of animals per test group associates the reward with each liner. For example, to compare learning ability in group A vs. group B, the reward must be associated with the white or brown liner in an equal number of mice in group A as that in group B. This is important to compensate for possible biases in the preference for one of the two patterns. Prepare a daily spreadsheet as shown in Table 1, with one line for each mouse. The experimenter will record independent and dependent variables in the columns, such as: Column 1: cage ID; Column 2: treatment (coded); Column 3: genotype (coded); Column 4: mouse ID; Column 5: reward pattern; Columns 6-22: correct/incorrect response; Columns 23 and 24: start and end time (Table 1). The sequence of the side of the rewarded vial changes every day by progressing one step, as described in Table 2.
Table 2. Example of sequence for reward presentation
Start training: Transfer the first mouse to the waiting zone, bait the vial with the pattern attributed to that animal, and open the door. The mouse should readily enter the reward zone and visit one of the vials. Check mouse behavior from the monitor without leaning over the arena when the mouse is in the reward zone. A choice is made when the mouse touches the vial with its snout or paws. If a correct choice is made, allow the mouse to consume the reward and cover the other vial with the sliding lid. If a wrong choice is made, quickly cover both vials with the sliding lids. If the mouse does not run back to the waiting zone, gently push it back with a plastic shovel identical to the sliding lids. Usually, mice learn to toggle between the zones during this first training day.
Once the mouse is back in the waiting zone, close the door, remove the pedestal and, if necessary, re-bait and re-place in the arena according to the scheduled side sequence; then, re-open the door. Proceed until 16 trials have been completed or 30 min has elapsed, whichever comes first. Usually, mice take 15-20 min to complete 16 trials during the first few training days, and only 5 min after 4-6 days. Mice not completing 16 trials after the first two training days suggest reduced motivation or excessive anxiety. If the source of the failure is not detected, these mice should be excluded from further testing.
When all mice in one batch have completed the training session, bring them back to the vivarium, weigh them, and give them food. Overnight food restriction of 1 g/mouse only applies if animals have not lost more than 10% of their original, pre-training body weight. After three days of training, food restriction is no longer necessary as mice will usually keep working for the pleasure; however, it can be re-introduced if animals fail to complete the 16-trial session over 30 min. For example, in our experience, food restriction can be stopped on day 4 without a subsequent reduction in performance of C57BL/6J mice (Hörnberg et al., 2020).
Follow the same procedure for the subsequent days until a pre-defined learning criterion is attained. A reliable criterion consists of attaining 8 consecutive correct responses for two days, which usually occurs after 5 days of training.
Rule reversal learning
The day following attainment of the first learning criterion, mice will start a contingency reversal training, i.e., the previously rewarded pattern will now be associated with a rejection response (sliding lid), while the previously non-rewarded pattern is now baited.
The first 2 trials of the reversal section are discovery trials: the mouse is allowed to explore both vials and consume the newly rewarded one. Discovery trials are meant to maintain the reward-seeking behavior even upon a first choice being made on the now empty vial that results in rejection. In addition, discovery trials prime the learning of the reversed contingency. These two trials are not counted during this first reversal training session, which continues with 16 trials as usual. As before, the learning criterion is 8 consecutive correct responses for two days in a row.
Reversal learning is usually attained after 7-10 days; however, it is useful to continue training all animals until all have attained criteria in order to compare the consistency of responses over time. If some mice have not learned after 12 days, the trial can be stopped.
The entire procedure, with its timeline, is graphically summarized in Figure 4.
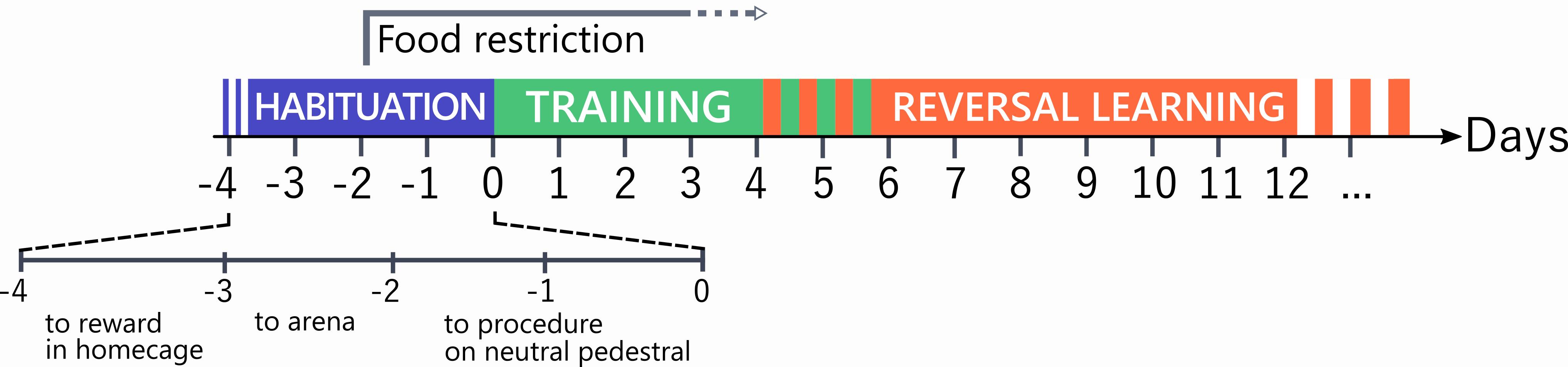
Figure 4. Schematic overview of the experimental timeline
Data analysis
Mice that, despite food restriction, do not complete 16 trials by the third test day are excluded from further testing. Depending on the goal of the experiment, several variables can be assessed. For example, a possible primary measure for the test is daily consecutive correct responses, with secondary measures including the absolute number of correct responses and a learning index, calculated as the difference in the number of correct responses between the first and last day of training. Statistical analysis should be performed using a one-way repeated-measures ANOVA when comparing within-group effects, or a two-way repeated-measures ANOVA when comparing the effect between groups or treatments for the primary measurement, or non-parametric versions of these tests if the data are not normally distributed. Other parameters can be examined using tests appropriate to the measure being examined, with consideration for violation of normality, repeated measures, and comparisons between multiple groups. An example of statistical analysis between transgenic mice and between treatment groups can be found in Hörnberg et al. (2020), Extended Data Figure 6.
Notes
Exclusion criteria
As in most cognitive tasks, food reinforcements may sometimes be insufficient to ensure the animal's undertaking of the course of actions leading to the reward (Dolivo, 2020); in other words, it may happen that some mice "do not work" to obtain the reward. The present protocol minimizes these events by providing the possibility of prolonging the habituation and shaping steps until all mice consistently seek and consume the reward since the training steps can be tailored to the individual needs of each animal. In comparison with tasks like a water maze, where the performance of animals is compared at a pre-established training time, the present protocol assesses the number of trials (i.e., the time) needed to achieve a given performance. Therefore, it may happen that all mice in the control group have reached the learning criterion, sometimes at 100% proficiency, while animals in the experimental group are still in their rule acquisition phase. However, for practical reasons, as described in the Procedure section, mice can be excluded from further training if they do not seek or consume baits in the habituation phase, do not willingly leave the waiting zone after two days of training, or do not complete a 16-trial session within 30 min on their third training day.
Animals
This protocol was practiced by six different experimenters and proved effective on several batches of mice of the strains C57BL/6J and FVB.129P2-Pde6b+ Tyrc-ch/AntJ (black-eyed FVB/N). The range of learning and reversal learning sessions needed to attain the criterion described herein refer to the C57BL/6J strain. FVB/N mice, in our experience, need more training to learn the rule, then perform at very high rates for the first contingency while performing more poorly in the reversal learning step. There is a minor concern about the visual acuity of pink-eyed mice, especially if carrying a retinal degeneration allele. The presented task is somatosensory in nature; nevertheless, we observed that at advanced stages of training, mice seem to switch to a visual strategy as they point straight to the reward pit before being in whisker contact with it.
While we only tested male mice, the protocol can also be applied to females; however, these should not be tested in the same sessions with males, as males will not work for the rewards if the arena contains traces of female odor.
Additional notes on procedures
Re-baiting the vials at a rapid and steady pace improves learning progression, possibly by maintaining the mouse's attention on the task. Therefore, the use of an automatic feeder could, in principle, improve the learning procedure; however, the conception and construction of an automated feeder that can change visual and/or somatesthetic aspects between trials is not trivial.
The use of different pairs of liners for the vials and pedestal (e.g., Velcro and PVC or metal vs. plastic wire mesh) would allow the assessment of flexibility in intra-dimensional shifts. To facilitate discrimination learning, we used liners that differed in both color and texture. However, the initial use of cues that only differ in color (e.g., white and black cellotape) would, in principle, allow the introduction of cues of different textures (e.g., white and black Velcro and sandpaper) suitable for an attentional set-shifting test. In comparison with the traditional setting based on odor-scented digging media, our paradigm has the advantage of avoiding the time-consuming step of training mice to dig and retrieve a solid reward. However, while we observed that trained mice clearly make their choice based on visual cues, we cannot exclude that separating visual from somatesthetic components may render the task initially more difficult, with consequent prolongation of the number of training days.
Acknowledgments
The original publication using this protocol received funding from EU-AIMS and AIMS-2-TRIALS, which are supported by the Innovative Medicines Initiatives from the European Commission, from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement no. 777394. This work was funded by a grant from the NCCR-Synapsy to Benjamin Boury-Jamot. This assay was developed at the Centre for Psychiatric Neuroscience, Department of Psychiatry at the Lausanne University Hospital by B.B.J. and F.M, and was first published in "Rescue of oxytocin response and social behaviour in a mouse model of autism" (Hörnberg et al., 2020). We thank P. Scheiffele for his support in publishing this protocol.
Competing interests
The authors declare no competing interests.
Ethics
All animal procedures were performed at the University of Lausanne in compliance with the Swiss National Institutional Guidelines on Animal Experimentation and were approved by the Lausanne Swiss Cantonal Veterinary Office Committees for Animal Experimentation with license no. 3024.
References
- Bissonette, G. B. and Powell, E. M. (2012). Reversal learning and attentional set-shifting in mice. Neuropharmacology 62(3): 1168-1174.
- Chang, Q. and Gold, P. E. (2003). Intra-hippocampal lidocaine injections impair acquisition of a place task and facilitate acquisition of a response task in rats. Behav Brain Res 144(1-2): 19-24.
- Colacicco, G., Welzl, H., Lipp, H. P. and Wurbel, H. (2002). Attentional set-shifting in mice: modification of a rat paradigm, and evidence for strain-dependent variation. Behav Brain Res 132(1): 95-102.
- Crusio, W. E. (2001). Genetic dissection of mouse exploratory behaviour. Behav Brain Res 125(1-2): 127-132.
- Deacon, R. M. and Rawlins, J. N. (2006). T-maze alternation in the rodent. Nat Protoc 1(1): 7-12.
- Devan, B. D. and White, N. M. (1999). Parallel information processing in the dorsal striatum: relation to hippocampal function. J Neurosci 19(7): 2789-2798.
- Dolivo, V. (2020). Mickey Mouse's negative affect facing mistakes. Learn Behav 48(1): 5-6.
- Goltstein, P. M., Reinert, S., Glas, A., Bonhoeffer, T. and Hubener, M. (2018). Food and water restriction lead to differential learning behaviors in a head-fixed two-choice visual discrimination task for mice. PLoS One 13(9): e0204066.
- Hebert, M., Bulla, J., Vivien, D. and Agin, V. (2017). Are Distal and Proximal Visual Cues Equally Important during Spatial Learning in Mice? A Pilot Study of Overshadowing in the Spatial Domain. Front Behav Neurosci 11: 109.
- Hörnberg, H., Perez-Garci, E., Schreiner, D., Hatstatt-Burkle, L., Magara, F., Baudouin, S., Matter, A., Nacro, K., Pecho-Vrieseling, E. and Scheiffele, P. (2020). Rescue of oxytocin response and social behaviour in a mouse model of autism. Nature 584(7820): 252-256.
- Izquierdo, A., Brigman, J. L., Radke, A. K., Rudebeck, P. H. and Holmes, A. (2017). The neural basis of reversal learning: An updated perspective. Neuroscience 345: 12-26.
- Jager, A., Dam, S. A., Van Der Mierden, S., Oomen, C. A., Arias-Vasquez, A., Buitelaar, J. K., Kozicz, T. and Glennon, J. C. (2020). Modulation of cognitive flexibility by reward and punishment in BALB/cJ and BALB/cByJ mice. Behav Brain Res 378: 112294.
- Kikusui, T., Winslow, J. T. and Mori, Y. (2006). Social buffering: relief from stress and anxiety. Philos Trans R Soc Lond B Biol Sci 361(1476): 2215-2228.
- Krackow, S., Vannoni, E., Codita, A., Mohammed, A. H., Cirulli, F., Branchi, I., Alleva, E., Reichelt, A., Willuweit, A., Voikar, V., Colacicco, G., Wolfer, D. P., Buschmann, J. U., Safi, K. and Lipp, H. P. (2010). Consistent behavioral phenotype differences between inbred mouse strains in the IntelliCage. Genes Brain Behav 9(7): 722-731.
- Lalonde, R. (2002). The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev 26(1): 91-104.
- Luo, J., Tan, J. M. and Nithianantharajah, J. (2020). A molecular insight into the dissociable regulation of associative learning and motivation by the synaptic protein neuroligin-1. BMC Biol 18(1): 118.
- Mechan, A. O., Wyss, A., Rieger, H. and Mohajeri, M. H. (2009). A comparison of learning and memory characteristics of young and middle-aged wild-type mice in the IntelliCage. J Neurosci Methods 180(1): 43-51.
- Packard, M. G. and McGaugh, J. L. (1992). Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci 106(3): 439-446.
- Packard, M. G. and McGaugh, J. L. (1996). Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem 65(1): 65-72.
- Sánchez-Moreno, J., Rodrigo, T., Chamizo, V. D. and Mackintosh, N. J. (1999). Overshadowing in the spatial domain. Animal Learn Behav 27(4): 391-398.
- Sanderson, D. J. and Bannerman, D. M. (2012). The role of habituation in hippocampus-dependent spatial working memory tasks: evidence from GluA1 AMPA receptor subunit knockout mice. Hippocampus 22(5): 981-994.
- Sanderson, D. J., Rawlins, J. N., Deacon, R. M., Cunningham, C., Barkus, C. and Bannerman, D. M. (2012). Hippocampal lesions can enhance discrimination learning despite normal sensitivity to interference from incidental information. Hippocampus 22(7): 1553-1566.
- Tait, D. S., Bowman, E. M., Neuwirth, L. S. and Brown, V. J. (2018). Assessment of intradimensional/extradimensional attentional set-shifting in rats. Neurosci Biobehav Rev 89: 72-84.
- Walker, E. L., Dember, W. N., Earl, R. W. and Karoly, A. J. (1955). Choice alternation. I. Stimulus vs. place vs. response. J Comp Physiol Psychol 48(1): 19-23.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Magara, F., Boury-Jamot, B. and Hörnberg, H. (2021). A Simple Spatial-independent Associative and Reversal Learning Task in Mice. Bio-protocol 11(15): e4108. DOI: 10.21769/BioProtoc.4108.
Category
Neuroscience > Nervous system disorders > Animal model
Neuroscience > Behavioral neuroscience > Learning and memory
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








