- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Quantitative Measurement of Mucolytic Enzymes in Fecal Samples
Published: Vol 11, Iss 6, Mar 20, 2021 DOI: 10.21769/BioProtoc.3956 Views: 5561
Reviewed by: Wathsala WijayalathRajesh ThippeshappaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Measurement of the Activity of Wildtype and Disease-Causing ALPK1 Mutants in Transfected Cells With a 96-Well Format NF-κB/AP-1 Reporter Assay
Tom Snelling
Nov 20, 2024 1601 Views
Fluorescence Polarization-Based High-Throughput Screening Assay for Inhibitors Targeting Cathepsin L
Keyu Guo [...] Shuyi Si
Jul 20, 2025 2274 Views
Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 477 Views
Abstract
The mucus layer in the gastrointestinal tract covers the apical surface of intestinal epithelial cells, protecting the mucosal tissue from enteric pathogen and commensal microorganisms. The mucus is primarily composed of glycosylated protein called mucins, which are produced by goblet cells, a type of columnar epithelial cells in the intestinal tract. Defective mucin barrier facilitates infection caused by enteric pathogen and triggers inflammation due to invasion of commensal or opportunistic pathogens into the intestinal epithelial mucosa. Several bacterial species in the gut produce enzymes that are capable of degradation of the mucus. Defective mucin production or increased abundance of mucolytic bacteria are clinically linked to inflammatory bowel disease. Measurement of mucolytic enzymes in the feces, therefore, can be implicated in clinical and experimental research on intestinal disorders. Here, we describe a step-by-step procedure for the measurement of the mucolytic enzyme activity in fecal samples.
Keywords: MucusBackground
The gastrointestinal tract (GI) is home for trillions of microorganisms which play diverse functions in the physiological processes (Sommer and Backhed, 2011). Commensal gut microbiota process undigested food, provide energy, nutrients and vitamins, activate the immune system, and prevent pathogens from infecting the intestinal mucosal tissue (Round and Mazmanian, 2009; Pickard et al., 2017). Despite these beneficial roles, gut commensal microorganisms may act as opportunistic pathogens when they get the opportunity to colonize intestinal epithelial barrier and invade into the mucosal tissue. However, a gel like mucus layer above the apical surface of the epithelial cells throughout the intestinal tract ensures physical separation of commensal microbes from the intestinal mucosal tissue and helps maintain intestinal homeostasis (Pullan et al., 1994; Linden et al., 2008; Atuma et al., 2011; Johansson et al., 2011; Juge, 2012). In the large intestine, mucus barrier is very thick, about 700 nm, and can be divided into two distinct layers – a thick outer layer and a thin inner layer (Johansson et al., 2008 and 2011). While the outer layer is nutrient rich, easy to be dislodged, and often colonized with anaerobic bacteria, the inner layer is firmly attached to the epithelial layer and is mostly sterile (Johansson et al., 2008 and 2011).
The mucus is primarily composed of glycoprotein called mucin, produced by goblet cells which are a type of columnar epithelial cells in the intestinal tract. Upon synthesis, mucin proteins are O-glycosylated or N-glycosylated with oligosaccharides and transported to the cell surface or secreted outside (McGuckin et al., 2011). Secretory mucins are heavily O-glycosylated and are homo-oligomerized via inter-molecular disulphide bond formed between the cysteine-rich D domain at the C and N terminus (Thornton et al., 2008). The major mucins in the outer layer that oligomerize to form the matrix are MUC2, MUC5AC, MUC5B, MUC6, and MUC19 (Thornton et al., 2008; McGuckin et al., 2011). The mucus is embedded with many antimicrobial peptides and immunoglobulins, which also keep the inner mucus layer sterile (McGuckin et al., 2011). On the other hand, oligosaccharides of the mucin serve as ligands and a source of nutrients for many anaerobic bacteria. Thus, several intestinal commensals as well as pathogens produce mucolytic enzymes, such as sulphatase, proteases, neuraminidases, α-glycosidase, β-glycosidase, β-galactosidase, fucosidase, β-N-acetylglucosaminidase, α-N-acetylgalactosaminidase, etc., to degrade mucins (Corfield et al., 1992; Linden et al., 2008; Johansson et al., 2011; Desai et al., 2013). Based on the diversity and complexity of mucin oligomers, cooperative actions are required from a number of enzymes as mentioned above for the degradation of mucins (Lombard et al., 2014). The major mucosa-associated bacteria belong to the phyla Proteobacteria, Actinobacteria, Firmicutes, Bacteroidetes, and Verrucomicrobia (Derrien et al., 2010; Tailford et al., 2015).
Enzymatic degradation of the mucus layer allows gut commensal bacteria or pathogen to breach the mucus barrier (Khan et al., 2020). Therefore, increased abundance of mucolytic bacteria facilitates enteric infection and is associated with inflammatory bowel diseases (IBD) such as Crohn’s disease and ulcerative colitis (Prizont, 1982; Carroll et al., 2010; Png et al., 2010; Hansson, 2012; Sheng et al., 2012). Thus, the level of mucus degrading enzymes in the colon could be a predictive marker for IBD. Measurement of mucolytic enzymes is also very useful in studies aimed at dissecting the mechanism of IBD pathogenesis in experimental or clinical settings.
Materials and Reagents
1.7 ml Posi-ClickTM Tubes (Denvelle, catalog number: C2170 )
96-well flat bottom plate (Thermo Scientific, catalog number: 12565136 )
Aluminium foil (Fisher Brand, catalog number: 01-213-100 )
Micropipette barrier tips (from 10 μl to 1,000 μl) (Genesee Scientific)
8-10 weeks-old C57Bl6/j mice
4-nitrophenol (Sigma-Aldrich, catalog number: 241326-50G )
4-nitrophenyl N-acetyl-β-D-glucosaminide (Sigma-Aldrich, catalog number: N9376 )
4-nitrophenyl α-D-galactopyranoside (Sigma-Aldrich, catalog number: N0877 )
4-nitrophenyl β-D-glucopyranoside (Sigma-Aldrich, catalog number: N7006 )
Fresh or -80 °C stored feces pellets
Acetone (EM Science, catalog number: AX0120-8 )
DNases (Sigma-Aldrich, catalog number: 11284932001 )
Lysozyme (Fisher BioReagentsTM, catalog number: BP535-1 )
Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M-0250 )
Methanol (Fisher Chemical, catalog number: A433P-4 )
p-nitrophenyl α-L-fucopyranoside (Sigma-Aldrich, catalog number: N3628 )
p-nitrophenyl β-D-xylopyranoside (Sigma-Aldrich, catalog number: N2132 )
PierceTM BCA protein assay kit (Thermo Scientific, catalog number: 23227 )
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541-500G )
Protease inhibitor cocktail tablet (Roche, catalog number: 26733200 )
Triton X-100 (Sigma-Aldrich, catalog number: T-9284 )
Trizma® hydrochloride (Sigma-Aldrich, catalog number: S8045-1KG )
4-Nitrophenyl (4NP) standard curve (see Recipes)
Mucolytic enzyme buffer (see Recipes)
Nitrophenyl-linked substrates and their corresponding mucolytic enzymes (see Recipes)
Equipment
A pair of sterile forceps
-80 °C freezer (Thermo Scientific)
Centrifuge (Thermo Scientific, Legend Micro 21R )
Ice making machine (Hoshizaki American Inc.)
Micropipette (from 10 μl to 1,000 μl) (Labnette)
Multi-channel pipette (300 μl) (Fisher Brand)
Sonicator with 3 mm tapered microtip (Branson Digital Sonifier, Model: 102C )
Spectrophotometer (TECAN, SPARK 10M)
Vortex Genie 2 (VWR Scientific Products)
Weighing balance (ADAM Equipment, PW124 )
Procedure
Collect 2-3 fecal pellets (approximately 50 mg) from each mouse into a tube.
Note: Feces can be stored at -80 °C until measurement.
Add 0.5 ml of ice-cold mucolytic enzyme buffer (Recipe 1) into the tube containing fecal pellets and gently vortex.
Note: Vertexing is not necessary if feces are not solid.
Sonicate samples using ultrasonic processor for 5 s with 35% amplitude and 3 mm tapered microtip on ice. Repeat ultrasonication following a 10 s interval for a total of 9 cycles or 45 s.
Centrifuge sonicated samples at 10,000 × g for 10 min at 4 °C.
Transfer ~400 μl supernatant into fresh 1.5 ml tubes and measure protein concentration.
Note: Supernatant can be stored at -80 °C, until measurement.
Adjust the protein concentration to 1 mg/ml by adding ice-cold mucolytic enzyme buffer.
Transfer 5 μl (5 μg protein) supernatant of a particular fecal sample into three wells (5 μl each) of a 96-well flat-bottom plate. Add 150 μl of a specific 10 mM nitrophenyl-based substrate (Recipe 2) prepared in ice-cold mucolytic enzyme buffer. For each enzyme tested, corresponding substrate is added into triplicate wells containing the same sample. These procedures are repeated when multiple samples are used.
Measure the absorbance at 405 nm in a plate reader at 37 °C at every 30 min interval.
Using a known concentration of 4-nitrophenol standard curve (Recipe 3), determine the individual mucolytic enzyme activity (Figure 1).
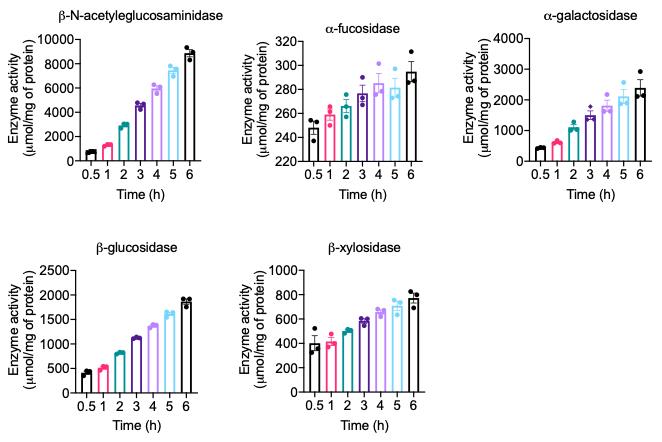
Figure 1. Time-dependent mucolytic enzymatic activity in mouse fecal samples. Fecal samples collected from healthy mouse were homogenized and processed as described in the protocol. The activity of indicated enzymes was measured in the fecal lysate following the procedure described in the protocol.
Recipes
Mucolytic enzyme buffer (pH 7.25)
Stock solution Working solution For 100 ml
1 M Tris 50 mM
1 M KCl 100 mM
1 M MgCl2 10 mM
Lysozyme 5-10 mg
12% Triton X-100 100 μl
DNases 5-10 mg
Protease inhibitor One tablet
Nitrophenyl-linked substrates and their corresponding mucolytic enzymes
Nitrophenyl-linked substrates Mucolytic enzymes 4-nitrophenyl α-D-galactopyranoside α-galactosidase Plant glycans 4-nitrophenyl N-acetyl-β-D-glucosaminide β-N-acetylglucosaminidas Mucin 4-nitrophenyl β-D-glucopyranoside β-glucosidase Plant glycans p-nitrophenyl α-L-fucopyranoside α-fucosidase Mucin p-nitrophenyl β-D-xylopyranoside β-xylosidase Plant glycans Note: We mentioned above nitrophenyl-linked substrates, which we use in the Figure 1.
4-Nitrophenol (4NP) standard curve
4-Nitrophenol (4NP) is an enzymatic product of p-nitrophenyl-linked substrates. The amount of 4NP produced during reaction of mucolytic enzymes with its substrate corresponds to the mucolytic enzyme activity (Recipe 2). 4NP provides yellow color and can be measured spectrophotometrically at 405 nm. Thus, a standard curve of 4NP can be used to measure mucolytic enzyme activity in a reaction mixture of mucolytic enzymes and its p-nitrophenyl-linked substrates.
Prepare a 100 mM 4NP (MW = 139.11) stock solution by dissolving 0.0139 g (13.9 mg) of 4NP in 1 ml of methanol.
Prepare a working stock solution of 1 mM 4NP (from 100 mM 4NP) for standard curve with mucin enzyme assay buffer.
Prepare a series of 4NP concentration ranging from 0 to 1,000 µM (or 0 to 1 mM) from the working stock (1 mM) prepared in mucin enzyme assay buffer.
Add 150 µl of each standard solution into a 96-well plate including blank and read the absorbance with a spectrophotometer at 405 nm at 37 °C.
Plot the standard curve with known standard concentration on the X-axis and absorbance on the Y-axis.
Acknowledgments
This protocol was adapted with minor modification from previous study published by Desai et al. (2016) and Khan et al. (2020). We would like to thank the UT Southwestern Animal Resource Center (ARC) for maintenance and care of our mouse colony. This work was supported by The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institute of Health (NIH) under Award Number R01DK125352, and Cancer Prevention and Research Institute of Texas (CPRIT) Individual Investigator Awards (RP200184), and UT Southwestern funding given to H.Z.
Competing interests
The authors declare no competing interests.
Ethics
This study was approved by the Institutional Animal Care and Use Committee (IACUC; approval No. 2016-101683), and was conducted in accordance with the IACUC guidelines.
References
- Atuma, C., Strugala, V., Allen, A. and Holm, L. (2001). The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 280(5): G922-929.
- Carroll, I. M., Chang, Y. H., Park, J., Sartor, R. B. and Ringel, Y. (2010). Luminal and mucosal-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Gut Pathog 2(1): 19.
- Corfield, A. P., Wagner, S. A., Clamp, J. R., Kriaris, M. S. andHoskins, L. C. (1992). Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect Immun 60(10): 3971-3978.
- Desai, M. S., Seekatz, A. M., Koropatkin, N. M., Kamada, N., Hickey, C. A., Wolter, M., Pudlo, N. A., Kitamoto, S., Terrapon, N., Muller, A., Young, V. B., Henrissat, B., Wilmes, P., Stappenbeck, T. S., Nunez, G. and Martens, E. C. (2016). A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167(5): 1339-1353 e1321.
- Derrien, M., van Passel, M. W., van de Bovenkamp, J. H., Schipper, R. G., de Vos, W. M. and Dekker, J. (2010). Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 1(4): 254-268.
- Hansson, G. C. (2012). Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol 15(1): 57-62.
- Johansson, M. E., Larsson, J. M. and Hansson, G. C. (2011). The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A 108 Suppl 1: 4659-4665.
- Johansson, M. E., Phillipson, J. M., Petersson, J., Velcich, A., Holm, L. and Hansson, G. C. (2008). The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 105(39): 15064-15069.
- Juge, N. (2012). Microbial adhesins to gastrointestinal mucus. Trends Microbiol 20(1): 30-39.
- Khan, S., Waliullah, S., Godfrey, V., Khan, M. A. W., Ramachandran, R. A., Cantarel, B. L., Behrendt, C., Peng, L., Hooper, L. V., Zaki, H. (2020). Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Sci Transl Med 12: eaay6218.
- Linden, S. K., Sutton, P., Karlsson, N. G., Korolik, V. and McGuckin, M. A. (2008). Mucins in the mucosal barrier to infection. Mucosal Immunol 1(3): 183-197.
- Lombard, V., Golaconda Ramulu, H., Drula, E., Coutinho, P. M. and Henrissat, B. (2014). The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42: D490-495.
- McGuckin, M. A., Linden, S. K., Sutton, P. and Florin, T. H. (2011). Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9(4): 265-278.
- Pickard, J. M., Zeng, M. Y., Caruso, R. and Nunez, G. (2017). Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev 279(1): 70-89.
- Png, C. W., Linden, S. K., Gilshenan, K. S., Zoetendal, E. G., McSweeney, C. S., Sly, L. I., McGuckin, M. A. and Florin, T. H. (2010). Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 105(11): 2420-2428.
- Prizont, R. (1982). Degradation of intestinal glycoproteins by pathogenic Shigella flexneri. Infect Immun 36(2): 615-620.
- Pullan, R. D., Thomas, G. A., Rhodes, M., Newcombe, R. G., Williams, G. T., Allen, A. and Rhodes, J. (1994). Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 35(3): 353-359.
- Round, J. L. and Mazmanian, S. K. (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9(5): 313-323.
- Sheng, Y. H., Hasnain, S. Z., Florin, T. H. and McGuckin, M. A. (2012). Mucins in inflammatory bowel diseases and colorectal cancer. J Gastroenterol Hepatol 27(1): 28-38.
- Sommer, F. and Backhed, F. (2013). The gut microbiota--masters of host development and physiology. Nat Rev Microbiol 11(4): 227-238.
- Tailford, L. E., Crost, E. H., Kavanaugh, D., Juge, N. (2015). Mucin glycan foraging in the human gut microbiome. Front Genet 6: 81.
- Thornton, D. J., Rousseau, K. and McGuckin, M. A. (2008). Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol 70: 459-486.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Khan, S. and Zaki, H. (2021). Quantitative Measurement of Mucolytic Enzymes in Fecal Samples. Bio-protocol 11(6): e3956. DOI: 10.21769/BioProtoc.3956.
- Khan, S., Waliullah, S., Godfrey, V., Khan, M. A. W., Ramachandran, R. A., Cantarel, B. L., Behrendt, C., Peng, L., Hooper, L. V., Zaki, H. (2020). Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Sci Transl Med 12: eaay6218.
Category
Immunology > Mucosal immunology > Digestive tract
Biochemistry > Protein > Activity
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









