- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Detection of Apoptosis-like Cell Death in Ustilago maydis by Annexin V-FITC Staining
Published: Vol 8, Iss 15, Aug 5, 2018 DOI: 10.21769/BioProtoc.2948 Views: 7636
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Genetic Tagging and Imaging of Proteins with iFAST in Candida albicans
Jonas Devos [...] Wouter Van Genechten
Oct 5, 2024 1484 Views
Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 477 Views
Abstract
Programmed cell death (PCD) guides the transition between key developmental stages in many organisms. PCD also remains an important fate for many organisms upon exposure to different stress conditions. Therefore, an insight into the progression of PCD during the execution of a biological phenomenon can yield significant details of the underlying mechanism. Apoptosis, as well as apoptosis-like programmed cell death, constitutes one of the forms of PCD in higher and lower eukaryotes respectively. Flipping of phosphatidylserine (PS) from the inner leaflet of the plasma membrane to the outer leaflet is among the different hallmarks of apoptosis/apoptosis-like PCD that marks the initiation of the said cell death event. This flipping can be detected through staining of the target cells using annexin V-FITC that binds specifically to PS. In Ustilago maydis the staining of the externally exposed PS by annexin V-FITC is difficult due to the presence of cell wall. The key to such staining, therefore, relies on the gentle removal of the cell wall without significantly altering the underlying plasma membrane architecture/topology. This protocol highlights the dependence of the PS staining on the extent of protoplastation of the stressed cells in Ustilago maydis.
Keywords: ApoptosisBackground
PS externalization constitutes one of the hallmarks of apoptosis-like PCD that can be detected very early (Martin et al., 1995). Hence the appearance of PS on the outer leaflet of the plasma membrane marks the onset of an apoptotic cell death phenomenon. Ustilago maydis is a biotrophic plant pathogen and infects host plant Zea mays. The lifecycle of U. maydis has been demonstrated to comprise of primarily two morphological forms namely the non-pathogenic haploid sporidial form and the pathogenic diploid filamentous form. The transition from the haploid to the diploid takes place through the mating of the compatible haploid strains on plant surfaces (Kahmann and Kamper, 2004). This leads to the generation of an infectious structure called appressoria that further penetrates the host plant as the filamentous form of the pathogen. Within the plant cells, the filamentous form of U. maydis again undergoes several transitions between morphologically distinct phases leading to sporulation. PCD has been demonstrated to play a significant role in the morphological transformations of different cell types primarily in higher eukaryotes (Buss et al., 2006; Suzanne and Steller, 2013). Also in some phytopathogenic fungi, PCD has been evidenced to be absolutely essential for the generation of appressoria (Veneault-Fourrey et al., 2006). Besides aiding in the switching between distinct morphological forms, PCD is also an end result of a harsh environmental stress response (Phillips et al., 2003). During penetration of the host plant the pathogens are exposed to a number of host defense response derived stress conditions. Among them, exposure to an increasingly oxidative environment is the most common. The primary reason behind this is the increased production of reactive oxygen species by the host in response to pathogen invasion (Torres et al., 2006). Assaying PCD in the fungal pathogen under each of these conditions mentioned can give significant insights into the pathogenic development as well as stress response of U. maydis. This protocol described the steps in detail to stain U. maydis sporidia under axenic culture conditions with annexinV-FITC to detect onset of any apoptosis-like cell death event upon exposure to adverse environmental conditions. However, it doesn’t include the staining of filamentous hyphae of U. maydis during its growth in-planta. Therefore this protocol is only applicable to the U. maydis sporidial cell suspension.
Materials and Reagents
- Plastic Petri-dishes (Tarsons, catalog number: 460020-90MM )
- 50 ml centrifuge tubes (Tarsons, catalog number: 546041 )
- 15 ml centrifuge tubes (Tarsons, catalog number: 546021 )
- 250 ml conical flasks (Fisher Scientific, FisherbrandTM, catalog number: 15429103 )
- Culture tubes (BOROSIL, catalog number: 9800U08 , 25 x 150 mm, 55 ml) with plastic caps (Tarsons, catalog number: 020070 )
- 10 ml disposable syringe (Dispo Van, Hindustan Syringes and Medical Devices Limited)
- 0.22 μm syringe filter (Sartorius, catalog number: 16532-K )
- Microscope slides (Polar Industrial, Blue Star, catalog number: PIC-1 , 75 x 25 mm)
- Cover slips (Blue Star, 22 mm square, 0.13-0.16 mm thick)
- Pipette tips (Tarsons, 0.2-10 μl, catalog number: 521000 ; 2-200 μl, catalog number: 521010 ; 200-1,000 μl, catalog number: 521020 )
- Inoculation loop
- Kimwipes (Tarsons, catalog number: 370080 , 11.17 x 21.3 cm)
- Sterile scalpel blades
- Organisms: Ustilago maydis solopathogenic strain SG200 (Kamper et al.,2006)
- Yeast extract powder (HiMedia Laboratories, catalog number: RM027 )
- Peptone, bacteriological (HiMedia Laboratories, catalog number: RM001 )
- Sucrose, A.R. (HiMedia Laboratories, catalog number: RM3063 )
- Potato Dextrose Broth (HiMedia Laboratories, catalog number: M403 )
- D-Sorbitol (Sigma-Aldrich, catalog number: S1876 )
- Tri-sodium citrate dihydrate (Merck, catalog number: 1.93619.0521 )
- Citric acid monohydrate (Merck, catalog number: 1.93011.0521 )
- Calcium chloride dihydrate (SRL Sisco Research Laboratories, catalog number: 0344317 )
- Tris (hydroxymethyl) aminomethane (Merck, Calbiochem, catalog number: 9210-OP )
- Hydrochloric acid about 37% (Merck, catalog number: 1.93001.0521 )
- Hydrogen peroxide 30% (Merck, catalog number: 1.93007.0521 )
- Lysing Enzymes from Trichoderma harzianum (Sigma-Aldrich, catalog number: L1412 )
- Annexin V-FITC apoptosis detection kit (Sigma-Aldrich, catalog number: APOAF )
- YEPSL media (see Recipes)
- Potato Dextrose Agar (PD agar) media (see Recipes)
- SCS buffer (see Recipes)
- Lysing enzymes in SCS (see Recipes)
- STC buffer (see Recipes)
- 10x binding buffer (see Recipes)
Equipment
- Pipette (0.5-10 μl, 2-20 μl, 20-200 μl, 100-1,000 μl)
- Weighing balance (Shimadzu, model: BL220H )
- Shaking incubator
- Double beam spectrophotometer (Hitachi High-Technologies, model: U-2910 )
- Light Microscope (ZEISS, model: Axioskop 40 )
- Confocal microscope (Leica Microsystems, model: Leica TCS-SP7 )
- Cold centrifuge (Thermo Fisher Scientific, Thermo ScientificTM, model: Sorvall RC 6 Plus , catalog number: 36-101-0816)
- Laminar air flow
- Autoclave
Procedure
- Growing the fungal cells
- Day 1: Inoculate 5 ml of YEPSL media (see Recipes) in a culture tube with the U. maydisSG200 strain from a PD-agar plate (take about a loopful of inoculum) and incubate overnight in a shaking incubator at 28 °C and 180 rpm.
Note: U. maydis usually forms a uniform lawn of cells in the PD agar plate if inoculated in higher density. However, when plated at lower density U. maydis single colonies are quite easily spotted on a PD agar plate. - Day 2: Inoculate 50 ml of YEPSL media in a 250 ml conical flask with the overnight grown culture at an OD600 of 0.2 and incubate at 28 °C and 180 rpm. Pellet down the cells by centrifugation in a 50 ml centrifuge tube at 4 °C, 1,070 x g for 5 min when the OD600 of the culture reaches 0.8 (it usually takes 2.5 to 3 h for the OD600 to reach 0.8).
- Day 1: Inoculate 5 ml of YEPSL media (see Recipes) in a culture tube with the U. maydisSG200 strain from a PD-agar plate (take about a loopful of inoculum) and incubate overnight in a shaking incubator at 28 °C and 180 rpm.
- Induction of apoptosis (Day 2)
Resuspend the pellet from the previous step in 50 ml YEPSL media supplemented with 10 mM H2O2 and incubate the resulting cell suspension in a shaking incubator for 1 h at 28 °C and 180 rpm. - Induction of protoplast formation (Day 2)
- Pellet H2O2-treated U. maydis cells by transferring the fungal culture to a 50 ml centrifuge tube followed by centrifugation at 4 °C, 1,070 x g for 10 min.
- Discard the supernatant and resuspend the resulting cell pellet in 25 ml SCS buffer (see Recipes) and centrifuge for 10 min at 4 °C, 1,070 x g.
- Discard the supernatant and resuspend the pellet in 2 ml of SCS buffer supplemented with 12.5 mg/ml Lysing enzyme (see Recipes) and incubate on ice.
- Start monitoring the protoplast formation from approximately 10 min of incubation in the Lysing enzyme solution. Take an aliquot of the cells every 3 min to observe protoplast formation under a light microscope at 40x magnification (Figure 1). Stop the reaction by adding 10 ml of SCS buffer to the cell suspension as soon as about 50% of the cells show early stage protoplastation.
- Centrifuge the resulting suspension at 4 °C, 470 x g for 15 min.
- Discard the supernatant and wash the pellet that now contains the early stage protoplasts 3 times with 10 ml SCS buffer and 1 time with 10 ml STC buffer (see Recipes) 10 min after every wash by centrifugation at 4 °C, 470 x g, 10 min.
Note: During the washing steps, resuspend cells well in SCS/STC buffer by gently rotating the cell suspension in 50 ml centrifuge tube by hand; use of pipette and microtip may damage the protoplasts.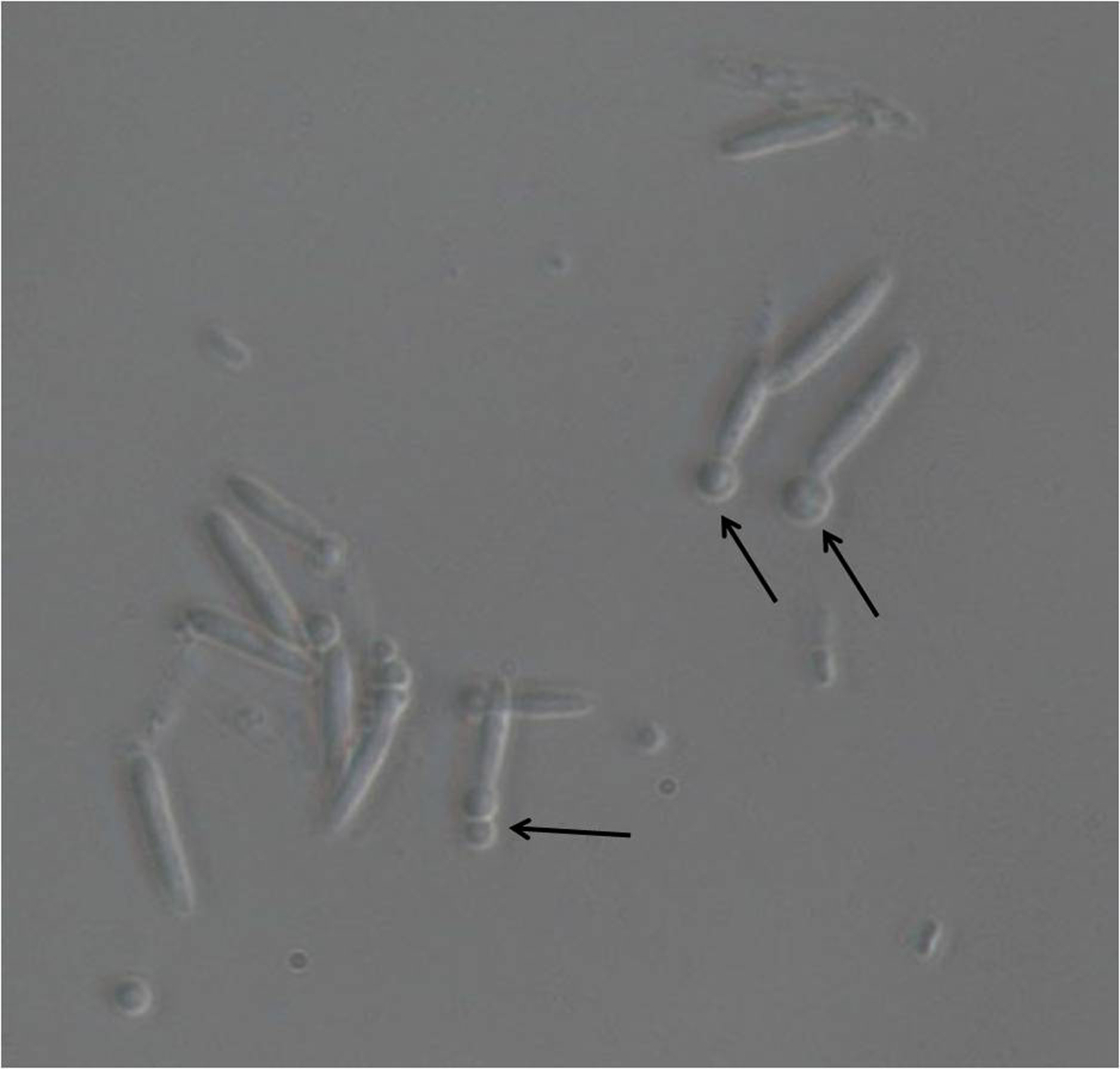
Figure 1. Light microscopic image of U. maydis sporidia after incubation with lysing enzymes. Magnification used is 40x. The emerging protoplasts are indicated with black arrows.
- Pellet H2O2-treated U. maydis cells by transferring the fungal culture to a 50 ml centrifuge tube followed by centrifugation at 4 °C, 1,070 x g for 10 min.
- Staining with Annexin V-FITC and Propidium Iodide (Day 2)
- Resuspend the pellet from the previous step in 500 μl of Annexin V-FITC binding buffer. (Prepare 1x binding buffer by diluting the 10x binding buffer (see Recipe 6) provided with the kit with autoclaved water). At this point the cells tend to clump (Figures 2B and 2D). Cut the edge of a 1 ml tip with a sterile scalpel blade to increase the cross sectional diameter of the edge. Try to dissolve the clumps by pipetting up and down the protoplast suspension using this tip.
Notes:- It is important to break the clumps as much as possible to ensure even staining of the protoplasts.
- Clumping of protoplast is a common problem and the degree of clumping has been shown to be directly dependent on the density of the cell suspension from where the protoplastation is induced (Wallin et al., 1974). Possibly exposed plasma membrane following removal of cell wall exhibits a sticky nature and can therefore interact with other protoplasts leading thereby to the formation of the protoplast aggregates or clumps.
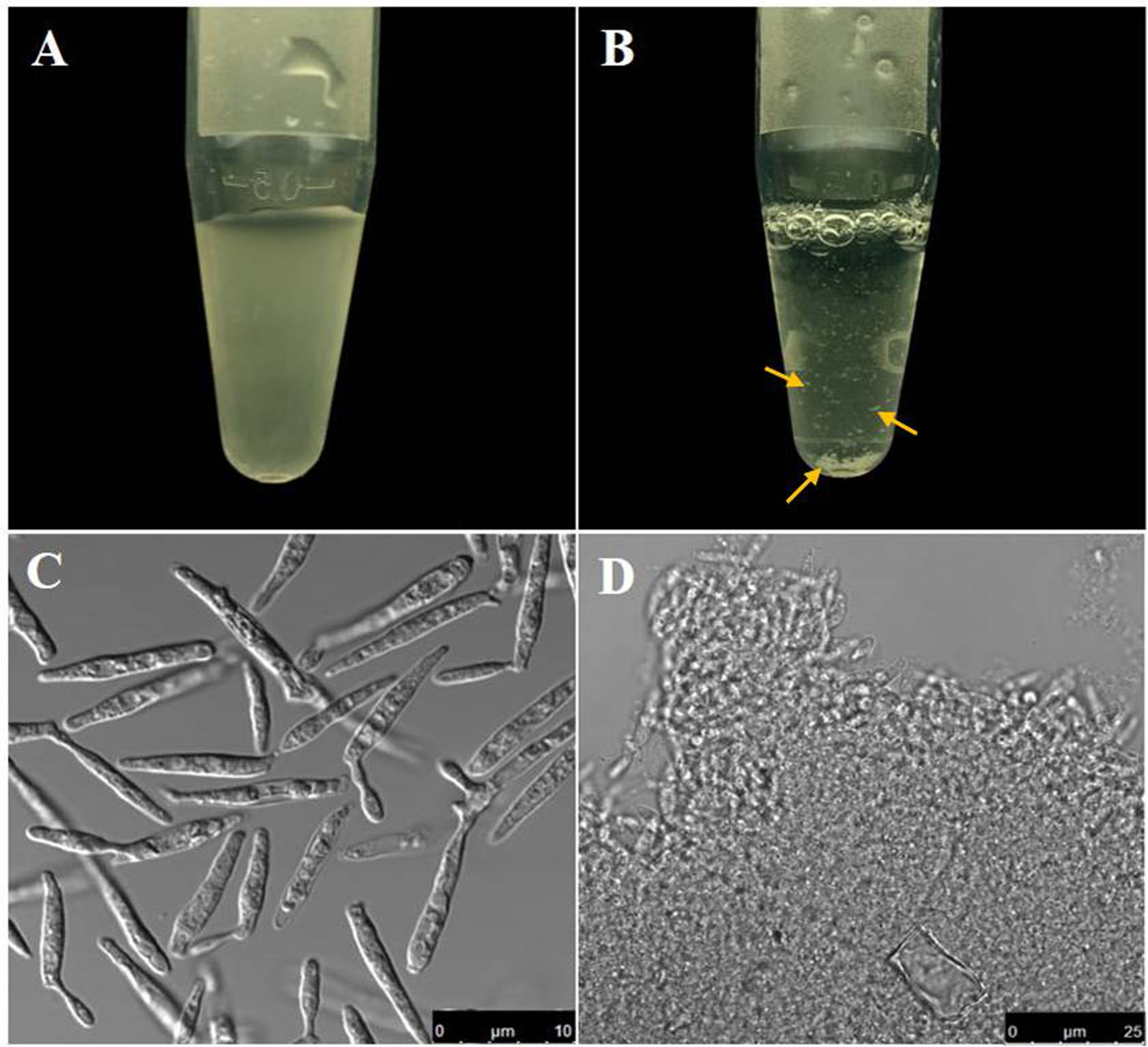
Figure 2. Comparative view of U. maydis cell suspension before and after protoplastation. A. Cell suspension containing U. maydis sporidia before protoplastation; B. Protoplast suspension (Note the clumps that formed due to removal of the cell wall); C-D. Bright field images of the cell suspensions in (A) and (B) respectively. Scale bars for (C) and (D) are indicated within the figure. Yellow arrows point the visible cell clumps noticed following protoplastation.
- It is important to break the clumps as much as possible to ensure even staining of the protoplasts.
- Add 0.5 μg/ml Annexin V-FITC conjugate and 2 μg/ml Propidium iodide to the cell suspension.
Note: Direct contact with PI should be avoided as it may cause inflammation of skin, irritation of eyes and respiratory tract. Gloves and protective glasses should always be worn while handling PI solution. - Incubate the cell suspension for 10 min in the dark at room temperature and then analyze the cells using a confocal microscope.
- Resuspend the pellet from the previous step in 500 μl of Annexin V-FITC binding buffer. (Prepare 1x binding buffer by diluting the 10x binding buffer (see Recipe 6) provided with the kit with autoclaved water). At this point the cells tend to clump (Figures 2B and 2D). Cut the edge of a 1 ml tip with a sterile scalpel blade to increase the cross sectional diameter of the edge. Try to dissolve the clumps by pipetting up and down the protoplast suspension using this tip.
- Preparing the microscope slides (Day 2)
- Clean the slides by rubbing well with kimwipes to remove any dirt-particle on the glass surface.
- Drop 5-6 μl of cell suspension in the middle of the glass surface.
- Carefully place a cover-slip over the drop of the cell suspension and seal it to the slide using a suitable coverslip sealant.
- Observe under a confocal microscope.
- Clean the slides by rubbing well with kimwipes to remove any dirt-particle on the glass surface.
Data analysis
Staining of PS externalization in U. maydis using annexin V-FITC is quite heterogeneous. In some cases, the fluorescence from FITC could be noticed primarily at the periphery of the protoplasts which is often seen in PS staining of higher eukaryotic cells. However, this peripheral localization of the fluorescence signal in case of U. maydis protoplasts has hardly any uniformity in terms of signal intensity throughout the periphery. For example, Figures 3A-3C show single (A and B) or multiple (C) intense spots at specific locations within the periphery of the stained protoplasts. On the contrary, some stained protoplasts show complete staining rather than a specific peripheral staining (Figures 3D-3F). Moreover, the complete staining is not always associated with late-stage apoptosis where the plasma membrane is already permeabilizedand protoplasts lack PI staining. Nevertheless, there are also instances where complete staining of protoplasts with both annexin V-FITC and PI could be noticed indicating a late stage of apoptosis (Figure 4). In none of the cases where peripheral FITC staining in U. maydis protoplasts could be seen, an internal PI staining could be noticed. Therefore, if protoplasts in U. maydis were stained in both the forms–peripheral staining as well as complete staining, it can be considered at the early stage of apoptosis. However, protoplasts that show both complete stainings with annexin V-FITC as well as PI should not be considered in their early stage of apoptosis. This is because these protoplasts might represent artifact associated with the protoplast preparation that led to the damage of the plasma membrane of sporidium and subsequent permeabilization to both PI and annexin V.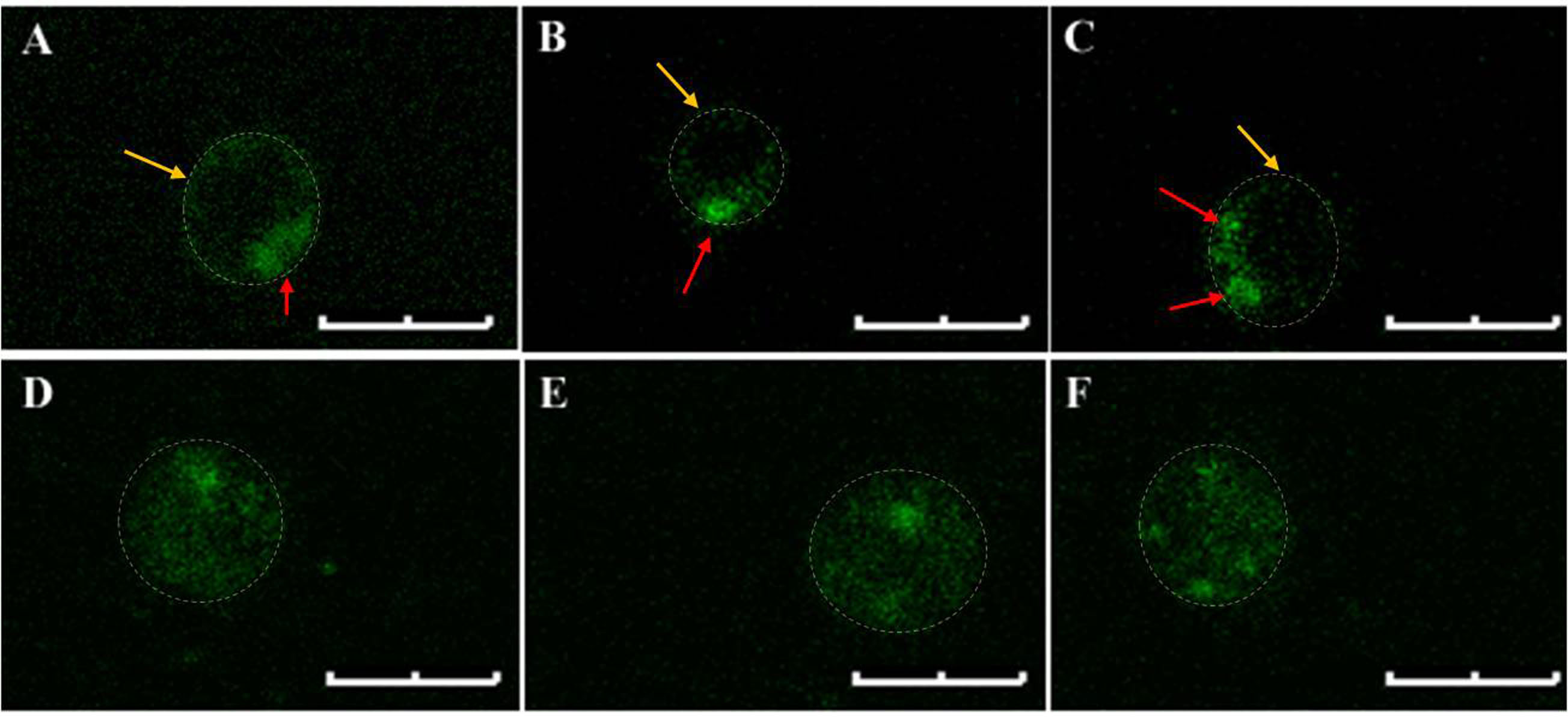
Figure 3. Annexin V-FITC stained U. maydis protoplasts. A-C. Typical peripheral localization of the fluorescence signals. Note the single or multiple intense spots (red arrows) in the otherwise uniformly stained periphery (Yellow arrows). D-F. Complete staining of the protoplasts. Note that no discrete peripheral staining is visible. The protoplast boundary is marked with dashed lines. Scale bars represent 4 μm. The settings for visualizing the fluorescence of FITC and Propidium Iodide are enlisted in Table 1.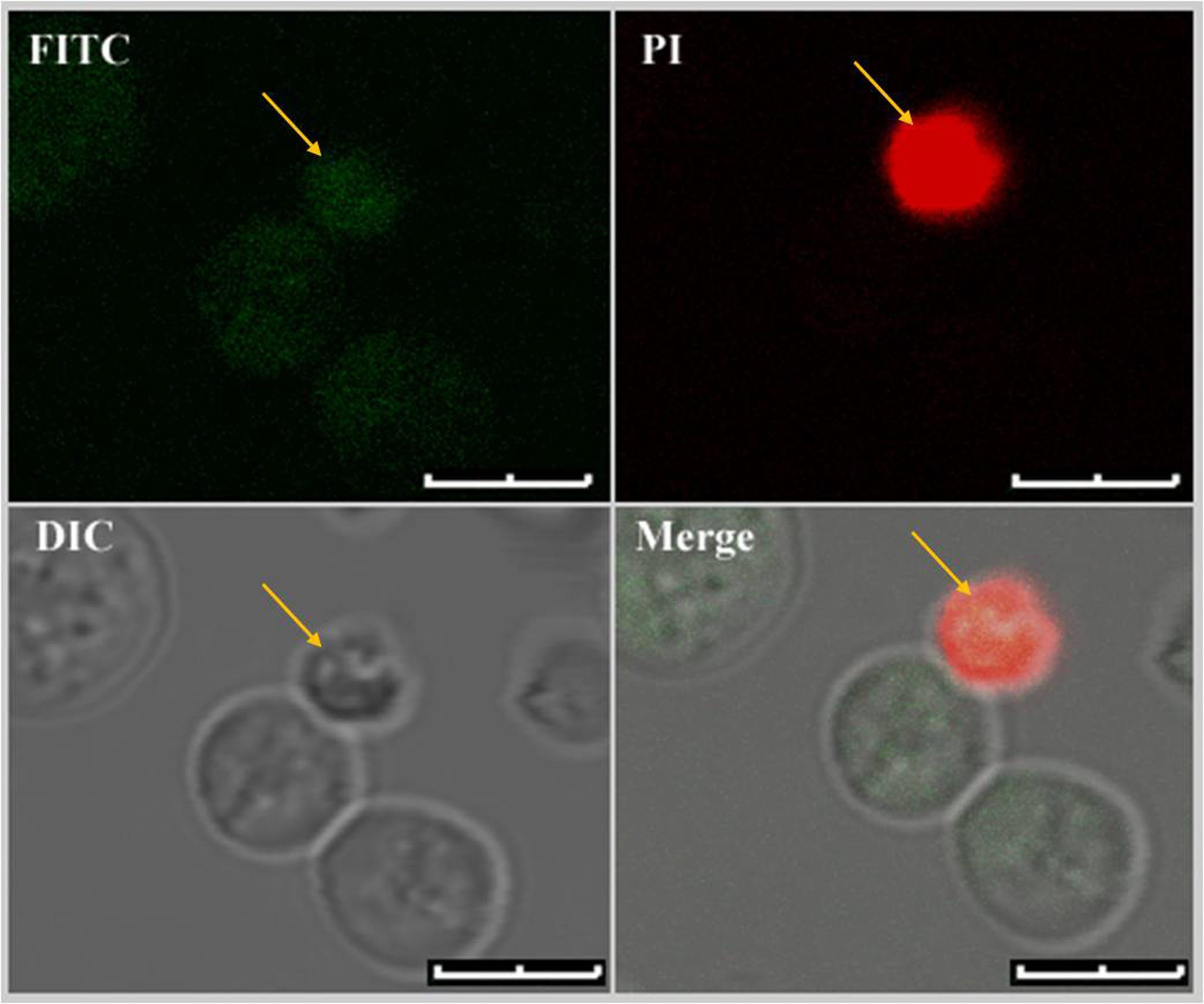
Figure 4. Dual staining of U. maydis protoplasts with Annexin V-FITC and PI. Only protoplasts with fully compromised plasma membrane showed fluorescence signals from both the fluorophores. Dual stained protoplast is marked with yellow arrows. Scale bars represent 4 μm.
Table 1. The laser settings for visualizing FITC and Propidium Iodide fluorescence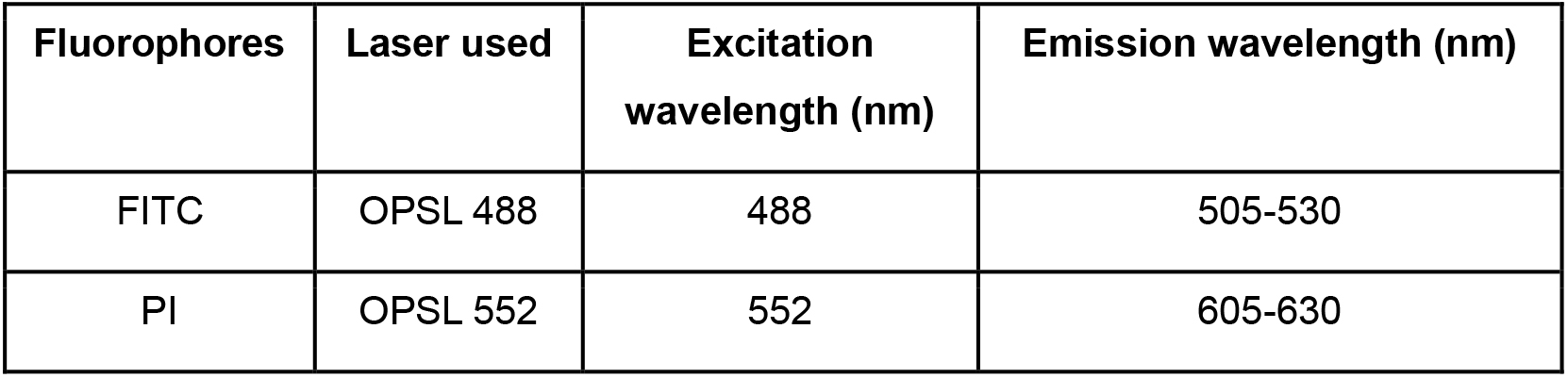
Notes
The limiting step for annexin V-FITC staining in U. maydis is the protoplastation condition. The cells are best stained with a typical peripheral staining marking the externalized PS only when the protoplasts have either just come out of the cell walls and not completely dissociated from them (STAGE I) or those that have freshly left out the cell wall (STAGE II). We believe that at this point the integrity of surface topology and architecture of the cells remain compatible for annexin V-FITC staining. The same samples when incubated for longer periods in the protoplast inducing buffer, either showed cytosolic stains or could not be stained at all. These are the final stage protoplasts and are completely separated from their respective cell walls for quite some time (STAGE III) (Figure 5).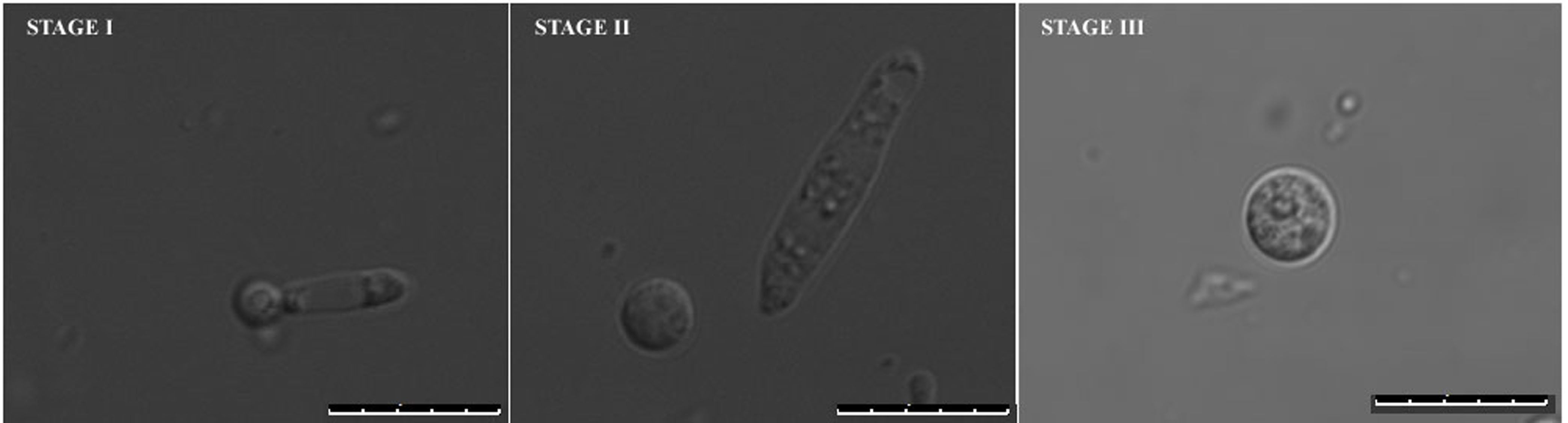
Figure 5. Different stages of protoplast formation in U. maydis. Stage I and II represent early stages protoplasts and Stage III represents late stage protoplasts with plasma membranes mostly incompatible for typical peripheral staining. Size bars represent 10 μm.
Recipes
- YEPSL media
Yeast extract: 10 g
Peptone, bacteriological: 4 g
Sucrose: 4 g
Dissolve in 1,000 ml of double distilled water, autoclave at 121 °C, 15 psi for 15 min - Potato Dextrose Agar (PD agar) media
- Dissolve 2.4 g of Potato Dextrose broth in double distilled water and adjust the volume to 100 ml
- To this add 2 g of agar powder and autoclave the resulting media at 15 psi for 15 min
- Following autoclaving cool down the media to about 50 °C and do the plating
- Dissolve 2.4 g of Potato Dextrose broth in double distilled water and adjust the volume to 100 ml
- SCS buffer
- To 100 ml of 20 mM Tri-sodium citrate dihydrate add D-sorbitol to a final concentration of 1 M
- Adjust pH with 1 M citric acid to 5.8, autoclave at 121 °C, 15 psi for 15 min and store at 4 °C
- To 100 ml of 20 mM Tri-sodium citrate dihydrate add D-sorbitol to a final concentration of 1 M
- Lysing enzymes in SCS (12.5 mg/ml)
- Dissolve 37.5 mg of Lysing enzymes in 3 ml of SCS buffer
- Pass the solution through a 0.22 μm syringe filter and keep on ice
- Dissolve 37.5 mg of Lysing enzymes in 3 ml of SCS buffer
- STC buffer
- To 50 ml of 10 mMTris (hydroxymethyl)aminomethane solution in distilled water add CaCl2 to a final concentration of 100 mM and D-sorbitol to a final concentration of 1 M
- Adjust the pH with 37% HCl to 7.5 and autoclave at 121 °C, 15 psi for 15 min and store at 4 °C
- To 50 ml of 10 mMTris (hydroxymethyl)aminomethane solution in distilled water add CaCl2 to a final concentration of 100 mM and D-sorbitol to a final concentration of 1 M
- 10x binding buffer
100 mM HEPES/NaOH, pH 7.5
1.4 M NaCl
25 mM CaCl2
Acknowledgments
This work was funded by DST-INSPIRE Faculty grant IFA13-LSPA16 and research grant from Bose Institute. The authors thank Mr. Asim Kumar Poddar for his assistance in obtaining confocal images that are presented here. This protocol is adapted from Mukherjee et al.(2017). The authors declare that there are no conflictsof interest.
References
- Buss, R. R., Sun, W. and Oppenheim, R. W. (2006). Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci 29: 1-35.
- Kahmann, R. and Kamper, J. (2004). Ustilago maydis: how its biology relates to pathogenic development. New Phytol 164(1): 31-42.
- Kamper, J., Kahmann, R., Bolker, M., Ma, L. J., Brefort, T., Saville, B. J., Banuett, F., Kronstad, J. W., Gold, S. E., Muller, O., Perlin, M. H., Wosten, H. A., de Vries, R., Ruiz-Herrera, J., Reynaga-Pena, C. G., Snetselaar, K., McCann, M., Perez-Martin, J., Feldbrugge, M., Basse, C. W., Steinberg, G., Ibeas, J. I., Holloman, W., Guzman, P., Farman, M., Stajich, J. E., Sentandreu, R., Gonzalez-Prieto, J. M., Kennell, J. C., Molina, L., Schirawski, J., Mendoza-Mendoza, A., Greilinger, D., Munch, K., Rossel, N., Scherer, M., Vranes, M., Ladendorf, O., Vincon, V., Fuchs, U., Sandrock, B., Meng, S., Ho, E. C., Cahill, M. J., Boyce, K. J., Klose, J., Klosterman, S. J., Deelstra, H. J., Ortiz-Castellanos, L., Li, W., Sanchez-Alonso, P., Schreier, P. H., Hauser-Hahn, I., Vaupel, M., Koopmann, E., Friedrich, G., Voss, H., Schluter, T., Margolis, J., Platt, D., Swimmer, C., Gnirke, A., Chen, F., Vysotskaia, V., Mannhaupt, G., Guldener, U., Munsterkotter, M., Haase, D., Oesterheld, M., Mewes, H. W., Mauceli, E. W., DeCaprio, D., Wade, C. M., Butler, J., Young, S., Jaffe, D. B., Calvo, S., Nusbaum, C., Galagan, J. and Birren, B. W. (2006). Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444(7115): 97-101.
- Martin, S. J., Reutelingsperger, C. P., McGahon, A. J., Rader, J. A., van Schie, R. C., LaFace, D. M. and Green, D. R. (1995). Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 182(5): 1545-1556.
- Mukherjee, D., Gupta, S., Saran, N., Datta, R. and Ghosh, A. (2017). Induction of apoptosis-like cell death and clearance of stress-induced intracellular protein aggregates: dual roles for Ustilago maydis metacaspase Mca1. MolMicrobiol 106(5): 815-831.
- Phillips, A. J., Sudbery, I. andRamsdale, M. (2003). Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc Natl Acad Sci U S A 100(24): 14327-14332.
- Suzanne, M. and Steller, H. (2013). Shaping organisms with apoptosis. Cell Death Differ 20(5): 669-675.
- Torres, M. A., Jones, J. D. and Dangl, J. L. (2006). Reactive oxygen species signaling in response to pathogens. Plant Physiol 141(2): 373-378.
- Veneault-Fourrey, C., Barooah, M., Egan, M., Wakley, G. and Talbot, N. J. (2006). Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science 312(5773): 580-583.
- Wallin, A., Glimelius, K. and Eriksson, T. (1974). The induction of aggregation and fusion of Daucus carota protoplasts by polyethylene glycol. Z Pflanzenphysiol 74(1): 64-80.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Mukherjee, D., Mitra, A. and Ghosh, A. (2018). Detection of Apoptosis-like Cell Death in Ustilago maydis by Annexin V-FITC Staining. Bio-protocol 8(15): e2948. DOI: 10.21769/BioProtoc.2948.
Category
Microbiology > Microbial cell biology > Cell staining
Cell Biology > Cell imaging > Confocal microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link