- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Tumorigenicity Assay in Nude Mice
Published: Vol 7, Iss 13, Jul 5, 2017 DOI: 10.21769/BioProtoc.2364 Views: 21979
Reviewed by: Antoine de MorreeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Modeling NOTCH1 driven T-cell Acute Lymphoblastic Leukemia in Mice
Agnieszka A. Wendorff and Adolfo A. Ferrando
May 20, 2020 6842 Views

Murine Pancreatic Islets Transplantation under the Kidney Capsule
Tatiana Jofra [...] Manuela Battaglia
Mar 5, 2018 11750 Views
Murine Hair Follicle Derived Stem Cell Transplantation onto the Cornea Using a Fibrin Carrier
Mindy Call [...] Ursula Schlӧtzer-Schrehardt
May 20, 2018 6619 Views
Abstract
Tumorigenicity refers to the ability of cultured cells to develop viable tumors in immune-deficient animals. The goal of this protocol is to illustrate tumorigenicity assay by subcutaneous tumor-cell-transplantation in nude mice. Target cells are transplanted to 6-week-old nude mice subcutaneously and the tumor growth is monitored over a period of observation or treatment. When tumor grows to a pre-determined size or by the end of the limited period, the nude mice will be euthanatized and the xenograft will be removed for further examination.
Keywords: TumorigenicityBackground
With high incidence and mortality, tumor is one of the leading causes of death and is a major public health problem. Extensive studies are conducted every year to explore tumor pathogenesis and anti-tumor therapy. In the process of cancer research, tumorigenicity assay in nude mice is a widely used experiment to monitor tumor growth in vivo (Giovanella et al., 1974). The most commonly used animal system for tumorigenicity assay is athymic nude mouse (Petricciani et al., 1973) where malignant cells can be transplanted either subcutaneously or subrenal-capsularly (van Meir, 1997). For cells with relatively low ability of tumorigenicity, take rate can be improved by irradiation (30-60 Gy) or implantation in Matrigel (Pretlow et al., 1991). Tumorigenicity assay provides a means of generating human cell derived tumor tissues for measurement of tumor cell malignancy and evaluation for anti-tumor drug efficacy.
Materials and Reagents
- 0.1-20 ml pipette tips (Eppendorf, catalog number: 22492012 )
- 5-200 ml pipette tips (Eppendorf, catalog number: 22492039 )
- 50-1,000 ml pipette tips (Eppendorf, catalog number: 22492055 )
- Cell culture disc (75-cm2) (Corning, catalog number: 430641 )
- Falcon 15 ml conical centrifuge tubes (Corning, catalog number: 430791 )
- Counting slides (Bio-Rad Laboratories, catalog number: 1450011 )
- Eppendorf tubes (1.5 ml) (Eppendorf, catalog number: 0030120086 )
- Tuberculin syringe (1 ml) (BD, catalog number: 300841 )
- BD 1 ml syringe (BD, catalog number: 309628 )
- BD PrecisionGlide needle (25 G, 0.5 x 40 mm) (BD, catalog number: 301808 )
- Female athymic nude mice (6-8 week old, Fourth Military Medical University, Experimental Animal Center, BALB/c)
- Phosphate buffered saline (PBS) pH 7.4 (Thermo Fisher Scientific, GibcoTM, catalog number: C10010500BT )
- Trypsin-EDTA (0.25%) (Thermo Fisher Scientific, GibcoTM, catalog number: 25200072 )
- Ethanol (Tianjin Fuyu Fine Chemical, catalog number: 20160916 )
- RPMI 1640 medium (Thermo Fisher Scientific, GibcoTM, catalog number: C11875500BT )
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10099141 )
- Penicillin-streptomycin (5,000 U/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15070063 )
- L-glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 25030081 )
- Complete 1640 medium (see Recipes)
Equipment
- 2-20 μl pipettes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 4641060N )
- 20-200 μl (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 4641080N )
- 100-1,000 μl (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 4641100N )
- Water-Jacketed CO2 incubators (Thermo Fisher Scientific, model: Model 3100 Series , catalog number: 3131)
- Clean bench (Thermo Fisher Scientific, model: HeraguardTM ECO )
- Centrifuge (Eppendorf, model: 5424 R )
- Automated cell counter (Bio-Rad Laboratories, model: T20TM )
- Microscope (Olympus, models: CX23 and BX53 )
- Caliper (Mitutoyo, catalog number: 530-118 )
Procedure
- Preparing cells for transplantation
- Cells were maintained in complete medium at 37 °C in 5% CO2.
- Trypsinize cells at 80-90% confluence (in exponential growth phase) and harvest cells with complete medium to a sterile centrifuge tube.
Note: Cells should be harvested in the exponential growth stage, which can greatly affect the viability of cell line transplantation. If the tumor viability is poor with a cell line xenograft, Matrigel can be used as injection mixture to provide an enriched stromal environment for engraftment. - Pellet cells at 60 x g for 5 min, remove supernatant and resuspend cells homogeneously in PBS.
Note: Cells should be resuspended into monoplast suspension for cell counting. - Measure and adjust cell concentration to 1-5 x 107/ml
Note: Ensure that cells suspension is mixed uniformly for accurate cell counting. - Transfer 5 x 106 cells and spin at 60 x g for 5 min for cell collection.
- Resuspend the pelleted cells in 0.2 ml PBS and move them into a sterile Eppendorf tube (1.5 ml) for injection.
- Cells were maintained in complete medium at 37 °C in 5% CO2.
- Subcutaneous injection of cells
- Fill injector (1 ml) with appropriate volume of cell suspension (0.2 ml), avoiding any bubbles to enter the syringe.
- Restrain the animal in an upright position by grasping the skin over the shoulders with the thumb and index finger, keeping the fore legs from pushing the syringe away (Figure 1).
- Disinfect the dorsal skin gently with 70%-ethanol-saturated cotton balls for 2-3 times (Figure 1).
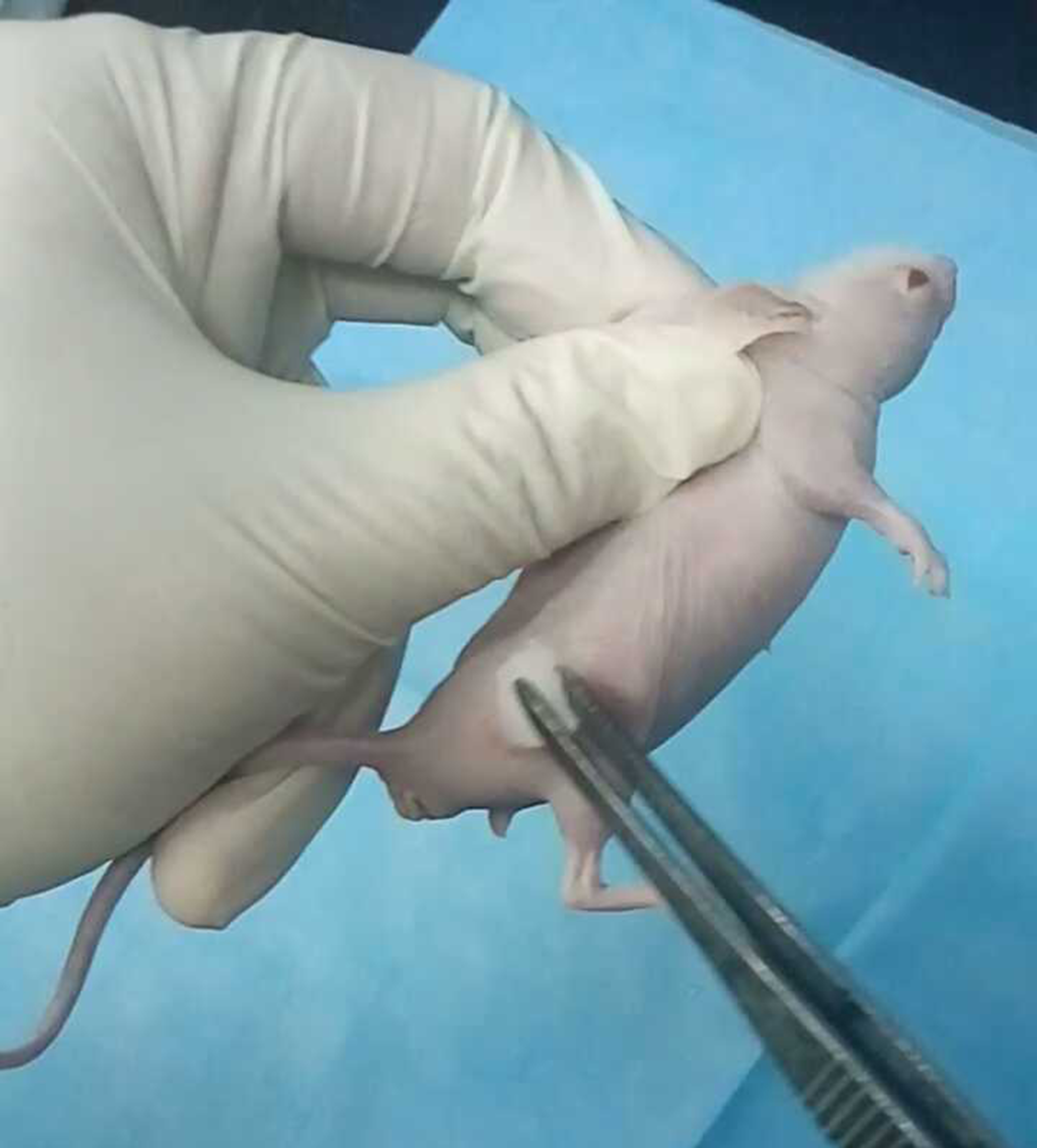
Figure 1. Disinfection of the nude mice dorsal skin. The nude mice were restrained by grasping the skin over the shoulders with left thumb and index finger. Disinfect the dorsal skin gently with 70%-ethanol-saturated cotton balls around the injection site for 2-3 times. - Gently insert the needle into the skin on the dorsal flank to reach the subcutaneous pocket (avoid the underlying muscle layer) (Figure 2A).
- Gently expel the contents of the syringe. (Figure 2B).
Note: If you inject accurately into the subcutaneous pocket, there will be no resistance to expel the contents of the syringe.
Figure 2. Subcutaneous injection of cell suspension into the nude mice. A. Inserting the needle into the skin on the dorsal flank to reach the subcutaneous pocket but not the underlying muscle layer; B. Expelling the contents of the syringe for the subcutaneous implant. - Withdraw the needle and place mice back into the cage.
Note: After injection, stay for a few seconds before pulling out the needle in case of leakage of cell suspension. - Monitor survival of nude mice and tumor growth 2-3 times a week, measuring the maximum (L) and minimum (W) length of the tumor using a slide caliper.
- Tumor volume can be calculated by the volume formula (Tomayko and Reynolds, 1989):
VT = 1/2(L x W x W)
where, L: the maximum length of the tumor; W: the minimum length of the tumor. - When tumor grows to a diameter of 150-200 mm3, remove the xenograft from sacrificed nude mice, weight and measure tumor size for tumor volume.
- Preserve the xenograft appropriately for histological or biochemical investigation if it is required.
- Fill injector (1 ml) with appropriate volume of cell suspension (0.2 ml), avoiding any bubbles to enter the syringe.
Data analysis
- Tumor size and weight of different groups are presented as the mean ± SD., which are adapted for tumor growth curve.
- Differences between means were assessed using Student’s t-test. P < 0.05 was considered to be statistically significant.
Notes
It is recommended to perform a small pilot study to determine the tumor take rate before the formal experiments. Additional cells and mice needed if the take rate is less than 100%.
Recipes
- Complete 1640 medium
10% FBS
1% penicillin-streptomycin
2 mM glutamine
Acknowledgments
This work was funded by NSFC grants 81602641 to Dr. Xiaodi Zhao.
References
- Giovanella, B. C., Stehlin, J. S. and Williams, L. J., Jr. (1974). Heterotransplantation of human malignant tumors in "nude" thymusless mice. II. Malignant tumors induced by injection of cell cultures derived from human solid tumors. J Natl Cancer Inst 52(3): 921-930.
- Petricciani, J. C., Kirchstein, R. L., Hines, J. E., Wallace, R. E. and Martin, D. P. (1973). Tumorigenicity assays in nonhuman primates treated with antithymocyte globulin. J Natl Cancer Inst 51(1): 191-196.
- Pretlow, T. G., Delmoro, C. M., Dilley, G. G., Spadafora, C. G. and Pretlow, T. P. (1991). Transplantation of human prostatic carcinoma into nude mice in matrigel. Cancer Res 51(14): 3814-3817.
- Tomayko, M. M. and Reynolds, C. P. (1989). Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 24(3): 148-154.
- van Meir, E. G. (1997). Identification of nude mice in tumorigenicity assays. Int J Cancer 71(2): 310.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Du, F., Zhao, X. and Fan, D. (2017). Tumorigenicity Assay in Nude Mice. Bio-protocol 7(13): e2364. DOI: 10.21769/BioProtoc.2364.
- Zhao, X. D., Lu, Y. Y., Guo, H., Xie, H. H., He, L. J., Shen, G. F., Zhou, J. F., Li, T., Hu, S. J., Zhou, L., Han, Y. N., Liang, S. L., Wang, X., Wu, K. C., Shi, Y. Q., Nie, Y. Z. and Fan, D. M. (2015). MicroRNA-7/NF-kappaB signaling regulatory feedback circuit regulates gastric carcinogenesis. J Cell Biol 210(4): 613-627.
Category
Cancer Biology > Cancer stem cell > Animal models > Cell invasion
Cell Biology > Cell Transplantation > Allogenic Transplantation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link