- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Allogeneic Transplantation of Testicular Hyperplasia in rag1 Mutant Zebrafish
Published: Vol 6, Iss 21, Nov 5, 2016 DOI: 10.21769/BioProtoc.1992 Views: 8110
Reviewed by: Raghuveer KavarthapuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Murine Pancreatic Islets Transplantation under the Kidney Capsule
Tatiana Jofra [...] Manuela Battaglia
Mar 5, 2018 11625 Views
Murine Hair Follicle Derived Stem Cell Transplantation onto the Cornea Using a Fibrin Carrier
Mindy Call [...] Ursula Schlӧtzer-Schrehardt
May 20, 2018 6571 Views
Abstract
Allogeneic organ transplantation is a powerful tool for clinical and basic research studies. However, the graft is often rejected by the host organism. Here, we describe a protocol that uses immunodeficient rag1 mutant zebrafish. These zebrafish escaped rejection, which made it possible to successfully transplant fragments of an allogeneic testis and testicular hyperplasia. This protocol can be used to amplify and maintain testicular hyperplasia grafts for several years (Kawasaki et al., 2016). The amplified hyperplasias are likely to be a good source of somatic and germ cells such as Sertoli cells and spermatogonial stem cells.
Background
Zebrafish have emerged as a tractable teleost genetic model for the study of vertebrate biology because several thousand mutants have been isolated by various genetic methods (Granato and Nüsslein-Volhard, 1996). Recently, this organism was used to study human diseases such as cancer (White et al., 2013). Although the incidence of spontaneous cancers is low, with many zebrafish eventually surviving cancer, allogeneic organ transplantation is a powerful tool, because many of the cancers are not syngeneic. Unfortunately, this method is not well developed. A previous study reported that zebrafish embryos accept cell grafts prior to the development of a mature immune system (Nicoli et al., 2007). However, it is difficult to successfully transplant grafts into embryos due to their minute size. For transplantation into adult zebrafish, sublethal γ-irradiation or immunosuppression with dexamethasone can block the rejection of the graft (Stoletov et al., 2007; White et al., 2008). However, it can be difficult to maintain cell grafts for long periods of time due to the short lifespans of recipients and the recovery of the immune response by 20 days after irradiation (Smith et al., 2010; Eguiara et al., 2011). Tissue grafts between identical clonal or inbred lines can survive without rejection (Kawasaki et al., 2010; Mizgirev and Revskoy, 2010; Shinya and Sakai, 2011).
T lymphocytes are central to the allograft response (Ingulli, 2010). The Recombination activating gene 1, 2 (rag1, Rag2) are important for immune function, because it creates double-stranded DNA breaks and is essential for V(D)J recombination, as well as for T and B cell function. rag1 mutant mice lack mature T and B cells, and they maintain allogeneic heart grafts for long periods of time (Zhang et al., 2006). By contrast, allogeneic transplantation has failed in rag1 mutant rats, probably due to the insufficient depletion of T and B cells (Ménoret et al., 2013). Hypomorphic rag2E450fs mutant zebrafish has been created, which have reduced V(D)J rearrangement and lymphocytes, and maintains various allogeneic cancer cells (Tang et al., 2014). Although rag1t26683 mutant zebrafish (hereafter rag1 mutant) have been isolated and they lack functional T and B cells (Wienholds et al., 2002; Petrie-Hanson et al., 2009), they were not used for transplantation. Our recent study reported that rag1 mutant zebrafish accept and maintain allogeneic testis organ and testicular hyperplasia grafts for long periods of time (Kawasaki et al., 2016). Here, we describe a protocol that uses immunodeficient rag1 mutant zebrafish for the subcutaneous transplantation of testis and testicular hyperplasia grafts.
Materials and Reagents
- 100 mm dish (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 263991 )
- Paper towels
- Surgical blades (FEATHER Safety Razor, catalog number: No. 25 )
- Aluminum foil
- rag1 mutant adult male zebrafish (Wienholds et al., 2002)
- L-15 medium (Sigma-Aldrich, catalog number: L5520-500ML )
- 25x phosphate-buffered saline (PBS)
- Ethyl p-aminobenzoate (Wako Pure Chemical Industries, catalog number: 057-03832 )
- Gentamicin (10 mg/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15710064 )
- Ethanol
- 10% ethyl p-aminobenzoate stock solution (see Recipes)
- 0.01% ethyl p-aminobenzoate working solution (see Recipes)
- 0.4x PBS containing 10 μg/ml gentamicin (see Recipes)
Equipment
- Stereoscopic microscope (OLYMPUS, model: SZ61 )
- Forceps (Style 5) (Dumont, catalog number: 0108-5-PO )
Procedure
- Subcutaneous transplantation
- Maintain all adult male recipients of rag1 mutant zebrafish without food 1 day prior to transplantation.
- Remove normal testes or spontaneous testicular hyperplasia from a donor fish as described in (Sakai, 2006), and cut to approximately 2-3 mm square containing the testis epithelium in L-15 medium.
- Place a paper towel in a 100 mm dish and pour a small amount of 0.4x PBS into the dish.
- Anesthetize the recipient zebrafish with 0.01% ethyl p-aminobenzoate.
- Place the anesthetized recipient fish into the 100 mm dish.
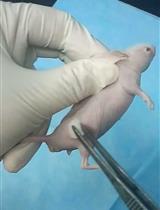
- Cover the head and tail of the recipient fish with wet paper towels (Figure 1A, Video 1).
- Descale the site of transplantation.
- Make an abdominal incision of approximately 5 mm in length with a razor blade.
- Insert the tips of a pair of forceps between the muscle and skin to prepare for the transplantation (Figure 1B, Video 1).
- Insert a fragment of the testis or testicular hyperplasia containing a small portion of adjacent tissue (Figure 1C, Video 1).
- Transfer the recipient zebrafish to a tank containing 0.4x PBS supplemented with 10 μg/ml gentamicin.
- Wrap the tank with aluminum foil to prevent the entry of light and incubate the tank at 26 °C for 4 days to promote wound healing.
- Rear the transplanted fish 4 days after surgery.

Figure 1. View of subcutaneous transplantation. A. Preparation of the recipient fish. The recipient fish is protected from drying by covering the head and tail with wet paper towels. B. Separation of the skin and muscle. The inserted forceps (arrow) separate the abdominal muscle from the skin of the recipient fish. C. View of the transplanted testis fragment. The testis fragment (arrowhead) is inserted into the treated area shown in (B). Scale bar = 5 mm.
Video 1. Procedures of subcutaneous transplantation
- Maintain all adult male recipients of rag1 mutant zebrafish without food 1 day prior to transplantation.
- Serial transplantation of testicular hyperplasia
- After growth of the testicular hyperplasia graft, anesthetize the recipient with 0.01% ethyl p-aminobenzoate.
- Remove the testicular hyperplasia and transfer to a 60 mm dish containing L-15 medium.
- Cut the graft into approximately ten fragments of equal size.
- To confirm the condition of the testicular hyperplasia graft, set aside several fragments for histology.
- Re-transplant the remaining fragments, which contain epidermis of the hyperplasia, as outlined in Procedure A: Subcutaneous transplantation.
- After growth of the testicular hyperplasia graft, anesthetize the recipient with 0.01% ethyl p-aminobenzoate.
Data analysis
We have performed a total of 156 cases of serial transplantation using 4 testicular hyperplasias, and growth of the transplanted grafts was observed in 149 cases, including data previously reported (Data is presented in Kawasaki et al., 2016). We have succeeded in maintaining a testicular hyperplasia more than 3 years at the longest (Data is presented in Kawasaki et al., 2016). Figure 2 shows the tree diagram of an additional testicular hyperplasia by serial transplantation for more than one year. However, malignant transformation and testis-ova arose very occasionally in the serial transplantation of testicular hyperplasias (Data is presented in Kawasaki et al., 2016). Therefore, histological observation of the grafted testicular hyperplasias should be performed in each generation to avoid undesired transformation.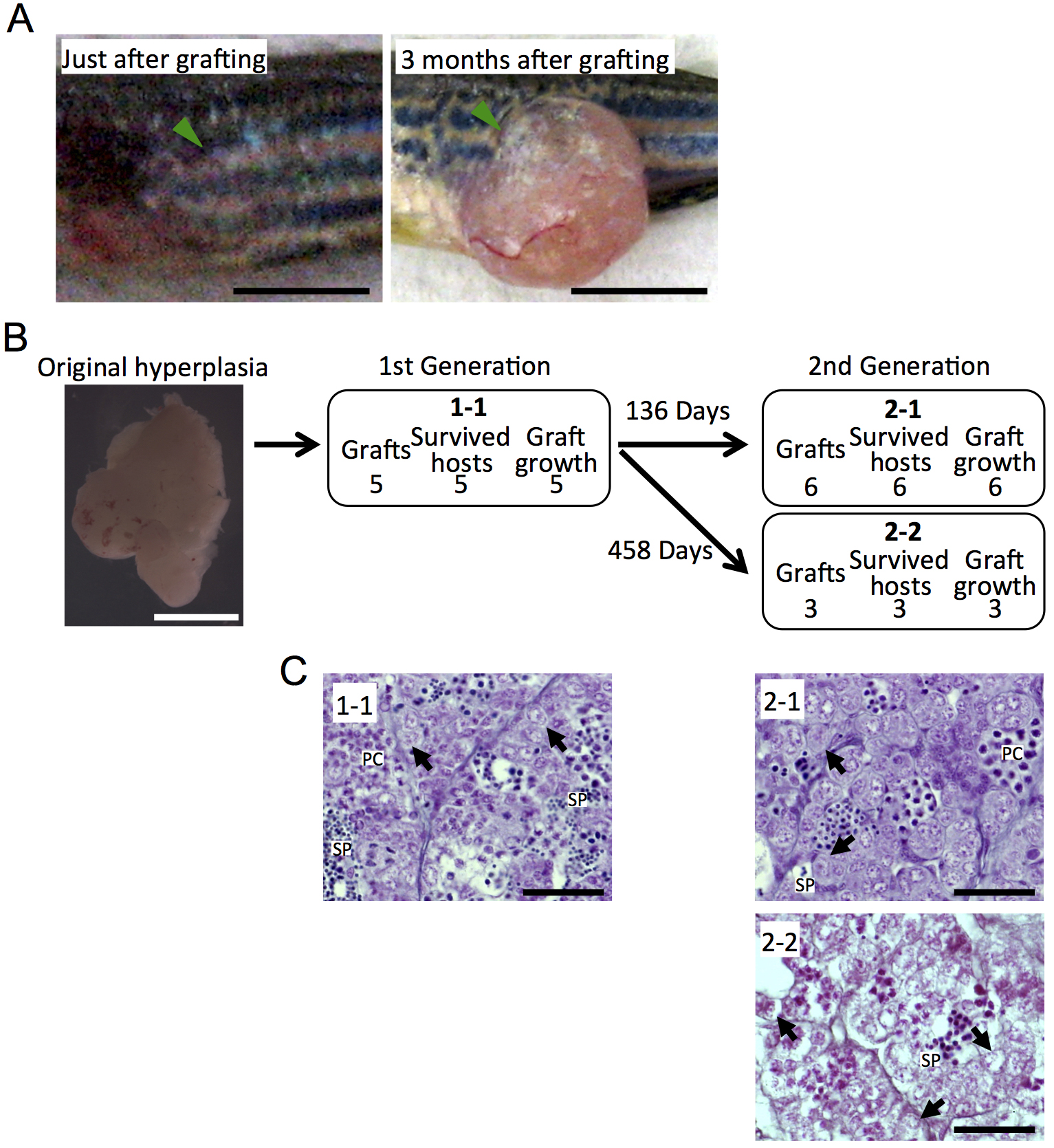
Figure 2. Maintenance of testicular hyperplasias by serial transplantation. A. Growth of the grafted fragment of a testicular hyperplasia. Arrowheads indicate the transplanted grafts. Scale bar = 5 mm. B. The tree diagram of serial transplantation of a testicular hyperplasia. Each box shows the number of the grafted fragments, the number of recipients survived for more than 1 month, and the number of the grown graft in each transplantation steps. Note that the fragment of the original testicular hyperplasia (the left photograph) grafted in the case of 1-1 was maintained for 458 days after the 1st transplantation, and that all grafts regrown in the 2nd transplantation. Scale bar = 5 mm. C. Histological observation of the grafted hyperplasia. Sections of each testicular hyperplasia correspond to the grafts shown in (B). Although the proportion of each stage of spermatogenic cells seemed to be changed, spermatogenesis was maintained through the serial transplantation. Arrows, spermatogonia; PC, spermatocytes; SP, sperm. Scale bar = 20 µm.
Recipes
- 10% ethyl p-aminobenzoate stock solution
Dissolve 5 g of ethyl p-aminobenzoate in 50 ml of ethanol - 0.01% ethyl p-aminobenzoate working solution
Dissolve 50 μl of 10% ethyl p-aminobenzoate stock solution in 50 ml of rearing water - 0.4x PBS containing 10 μg/ml gentamicin
Combine 16 ml of 25x PBS and 1 ml of gentamicin stock solution (10 mg/ml) in 983 ml of autoclaved rearing water
Acknowledgments
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sport, Science and Technology, Japan (Grant Nos. 23570260, 25251034, and 25114003). This protocol was adapted from the previous work (Kawasaki et al., 2016)
References
- Eguiara, A., Holgado, O., Beloqui, I., Abalde, L., Sanchez, Y., Callol, C. and Martin, A. G. (2011). Xenografts in zebrafish embryos as a rapid functional assay for breast cancer stem-like cell identification. Cell Cycle 10(21): 3751-3757.
- Granato, M. and Nüsslein-Volhard, C. (1996). Fishing for genes controlling development. Curr Opin Genet Dev 6(4): 461-468.
- Ingulli, E. (2010). Mechanism of cellular rejection in transplantation. Pediatr Nephrol 25(1): 61-74.
- Kawasaki, T., Saito, K., Shinya, M., Olsen, L. C. and Sakai, N. (2010). Regeneration of spermatogenesis and production of functional sperm by grafting of testicular cell aggregates in zebrafish (Danio rerio). Biol Reprod 83(4): 533-539.
- Kawasaki, T., Siegfried, K. R. and Sakai, N. (2016). Differentiation of zebrafish spermatogonial stem cells to functional sperm in culture. Development 143(4): 566-574.
- Ménoret, S., Fontanière, S., Jantz, D., Tesson, L., Thinard, R., Rémy, S., Usal, C., Ouisse, L. H., Fraichard, A. and Anegon, I. (2013). Generation of Rag1-knockout immunodeficient rats and mice using engineered meganucleases. FASEB J. 27(2): 703-711.
- Mizgirev, I. and Revskoy, S. (2010). Generation of clonal zebrafish lines and transplantable hepatic tumors. Nat Protoc 5(3): 383-394.
- Nicoli, S., Ribatti, D., Cotelli, F. and Presta, M. (2007). Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res 67(7): 2927-2931.
- Petrie-Hanson, L., Hohn, C. and Hanson, L. (2009). Characterization of rag1 mutant zebrafish leukocytes. BMC Immunol 10: 8.
- Sakai, N. (2006). In vitro male germ cell cultures of zebrafish. Methods 39(3): 239-245.
- Shinya, M. and Sakai, N. (2011). Generation of highly homogeneous strains of zebrafish through full sib-pair mating. G3 (Bethesda) 1(5): 377-386.
- Smith, A. C., Raimondi, A. R., Salthouse, C. D., Ignatius, M. S., Blackburn, J. S., Mizgirev, I. V., Storer, N. Y., de Jong, J. L., Chen, A. T., Zhou, Y., Revskoy, S., Zon, L. I. and Langenau, D. M. (2010). High-throughput cell transplantation establishes that tumor-initiating cells are abundant in zebrafish T-cell acute lymphoblastic leukemia. Blood 115(16): 3296-3303.
- Stoletov, K., Montel, V., Lester, R. D., Gonias, S. L. and Klemke, R. (2007). High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc Natl Acad Sci U S A 104(44): 17406-17411.
- Tang, Q., Abdelfattah, N. S., Blackburn, J. S., Moore, J. C., Martinez, S. A., Moore, F. E., Lobbardi, R., Tenente, I. M., Ignatius, M. S., Berman, J. N., Liwski, R. S., Houvras, Y. and Langenau, D. M. (2014). Optimized cell transplantation using adult rag2 mutant zebrafish. Nat Methods 11(8): 821-824.
- White, R. M., Sessa, A., Burke, C., Bowman, T., LeBlanc, J., Ceol, C., Bourque, C., Dovey, M., Goessling, W., Burns, C. E. and Zon, L. I. (2008). Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2(2): 183-189.
- White, R., Rose, K. and Zon, L. (2013). Zebrafish cancer: the state of the art and the path forward. Nat Rev Cancer 13(9): 624-636.
- Wienholds, E., Schulte-Merker, S., Walderich, B. and Plasterk, R. H. (2002). Target-selected inactivation of the zebrafish rag1 gene. Science 297(5578): 99-102.
- Zhang, Y., Kirken, R. A., Furian, L., Janczewska, S., Qu, X., Hancock, W. W., Wang, M., Tejpal, N., Kerman, R., Kahan, B. D. and Stepkowski, S. M. (2006). Allograft rejection requires STAT5a/b-regulated antiapoptotic activity in T cells but not B cells. J Immunol 176(1): 128-137.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kawasaki, T. and Sakai, N. (2016). Allogeneic Transplantation of Testicular Hyperplasia in rag1 Mutant Zebrafish. Bio-protocol 6(21): e1992. DOI: 10.21769/BioProtoc.1992.
Category
Stem Cell > Germ cell > Spermatogonial Stem Cell
Cell Biology > Cell Transplantation > Allogenic Transplantation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link