- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measuring Rat Serum Osmolality by Freezing Point Osmometry
Published: Vol 6, Iss 19, Oct 5, 2016 DOI: 10.21769/BioProtoc.1950 Views: 14367
Reviewed by: Soyun KimEmmanuelle BerretAntoine de Morree
Abstract
Blood serum or plasma osmolality is the measure of electrolyte to water balance in the body’s circulation, and is tightly regulated in physiological states in order to maintain normal levels of serum solute (Bourque, 2008). Osmolality is defined as the number of osmoles of solute per kg of water (mOsm/kg) (Dufour, 1993) and can be measured using different techniques that rely on the colligative properties of the solution. The most commonly used in lab settings are vapour pressure and freezing point osmometry, which are relatively quick and easy to perform. Freezing point osmometry is preferred because it is insensitive to volatile compounds, such as alcohol, that may be present in the solution. Measurement of serum or plasma osmolality is clinically relevant for a number of conditions and diseases, including hypernatremia, diabetic ketoacidosis, and the syndrome of inappropriate antidiuresis (Ellison, 2013; Lupsa and Inzucchi, 2013; Reddi, 2013). In this protocol, we describe the measurement of serum osmolality in rats using the freezing point osmometry technique as originally outlined in our previous study of osmoregulatory perturbations in sepsis (Stare et al., 2015).
Keywords: OsmolalityMaterials and Reagents
- Underpad (AMG Medical, catalog number: 760-372 )
- 21 G x 1 ½ in. needle (BD, catalog number: 305167 )
- 5 ml syringe (BD, catalog number: 309646 )
- 1.5 ml microcentrifuge tube (Diamed, catalog number: DIATEC610-3169 )
- 1,000 μl pipette tips (Diamed, catalog number: DIATEC520-6753 )
- Kimwipes (Thermo Fisher Scientific, Fisher Scientific, catalog number: 06-666A )
- 1 rat (Rattus rattus; 100-300 g)
- Isoflurane (CDMV, catalog number: 19417 )
- 100% oxygen
- 290 mOsm/kg control solution (ClinitrolTM 290 Reference Solution) (Advanced Instruments, catalog number: 3MA029 )
Equipment
- Forceps (Dumont #7b forceps) (Fine Science Tools, catalog number: 11270-20 )
- Veterinary gas anesthesia machine (Dispomed, model: 975-3300-000MRI )
- Induction chamber (VetEquip, model: 941444 )
- 1,000 μl pipette (Gilson, PipetmanTM, model: F123602 )
- Nosecone connected to Bain circuit (VetEquip, model: 921463 )
- Centrifuge (Spectrafuge 24D) (Labnet International, model: C2400 )
- Vortex (Vortex Genie 2) (Scientific Industries, model: SI-0236 )
- Freezing point osmometer (Advanced Instruments, model: 3320 Micro-Osmometer )
- Ease-Eject 20-Microliter sampler (Advanced Instruments, model: 3M0825 )
- Sampler plunger wire (Advanced Instruments, catalog number: 3M0828 )
- Sampler tips and chamber cleaners (Advanced Instruments, model: 3MA800 )
Procedure
- Cardiac puncture
This section describes the cardiac puncture method for acquiring and handling a blood sample in preparation for measuring serum osmolality (see Note A1).- Deep anesthesia will take approximately 10 min overall. Place the rat into the induction chamber. Adjust the oxygen flow rate to 1.5-2 L/min, and the isoflurane vapourizer to 5%.
- Once the rat has stopped moving for several minutes (2-4 min), it can be transferred onto the working surface and placed on the mask attached to a Bain circuit.
- Place the rat parallel to self in the supine position, with the head towards your non-dominant hand, and tail towards your dominant hand. Adjust the oxygen flow rate to 0.4-0.8 L/min and continue at 3-5% isoflurane.
- Verify that it is deeply anesthetized by using the tweezers to pinch the toes. The rat should not respond. Maintain the isoflurane at 3-5% (see Note A2).
- Test the plunger on the 5 ml tube, making sure that it moves smoothly. Leave the plunger slightly retracted. Attach the 21 G 1 ½ in. needle.
- Using your non-dominant hand, gently press the forelimbs down on the table and inwards into the body so that the claws face towards its hind legs and the forelimbs are parallel to the chest. This 'squeezing' of the forelimbs into the body will lift up the rib cage providing easier access to the heart.
- With your dominant hand, insert the needle to the anatomical left of the xiphoid process at a 30° angle (upwards from the chest) and slowly push it into the chest while maintaining a gentle negative pressure with the plunger (see Note A3). You may feel a resistance when you reach the heart, but this is not always the case.
- Once you have reached the heart, a small amount of blood will enter the syringe. Release the negative pressure. Let go of the forelimbs, and use your non-dominant hand to stabilize the syringe. Use your dominant hand to slowly pull back on the syringe.
- Once you have collected your desired amount of blood (see Note A4), gently apply a small positive pressure by pushing the plunger in, and pull the needle out. A bit of positive pressure will prevent hemolysis during retraction of the needle (see Note A5).
- Immediately transfer the blood in the syringe into a 1.5 ml microcentrifuge tube. To do so, remove the needle from the syringe and gently expel the blood into the tube. The blood will have begun to coagulate and if this step is done too quickly, it will cause hemolysis.
- Leave at room temperature (RT, ~22 °C) for 30-60 min, or place the tube on ice in an insulated foam container with the lid closed for a minimum of 30 min and up to 9 h (see Note A6 and Representative data).
- Follow up with a recommended physical method of euthanization (e.g., pneumothorax, cervical dislocation) as per your institution’s animal use guidelines.
- Deep anesthesia will take approximately 10 min overall. Place the rat into the induction chamber. Adjust the oxygen flow rate to 1.5-2 L/min, and the isoflurane vapourizer to 5%.
- Serum separation
This section describes the blood processing necessary to prepare the serum sample for osmolality measurement.- Centrifuge the blood sample at 6,000 x g for 5 min. The resultant thick and dark red pellet will contain the haemocytes. The clear, off-white-to-yellow supernatant layer will contain the serum. 1.5 ml of blood provides approximately 0.5-0.7 ml of serum (see Notes B1 and B2).
- Pipette the supernatant into a fresh microcentrifuge tube. Be sure not to disturb the pellet when collecting the serum.
- Store at 4 °C until ready for further processing, making sure that the lid is completely closed to prevent evaporation.
- Centrifuge the blood sample at 6,000 x g for 5 min. The resultant thick and dark red pellet will contain the haemocytes. The clear, off-white-to-yellow supernatant layer will contain the serum. 1.5 ml of blood provides approximately 0.5-0.7 ml of serum (see Notes B1 and B2).
- Osmolality measurement
This section starts by verifying the reliability of the osmometer by measuring a solution with a known osmolality value (290 mOsm/kg control solution), followed by the method of measuring serum osmolality.- Make sure that your osmometer has been recently calibrated following the manufacturer’s guidelines, and is in good working order.
- This is a quality control step to test the osmometer (see Notes C1 and C2).
- Attach a sampler tip to the sampler. Use the sampler to draw 20 μl of the 290 mOsm/kg control solution.
- Wipe the outside of the sampler tip dry with a Kimwipe, including the tip perimeter. Blot excess sample so that the meniscus is in line with the tip or slightly concave (see Note C3).
- Insert the sampler into the cooling chamber. The measurement will take approximately 1 min.
- When the measurement has been completed, pull out the sampler, and remove and discard the sampler tip (see Note C4).
- Dry the sampler plunger wire with a Kimwipe.
- Clean the cooling chamber with a chamber cleaner as per the manufacturer’s instructions
- Repeat steps C2 a-f two more times.
- Attach a sampler tip to the sampler. Use the sampler to draw 20 μl of the 290 mOsm/kg control solution.
- Check for any residue at the bottom of your serum sample. Should residue be present, centrifuge the sample again at 6,000 x g for 2.5 min, and pipette the serum into a fresh microcentrifuge tube.
- Vortex the sample for 5 sec.
- Measure the serum osmolality using the same technique described in step C2, briefly vortexing (~3 sec) the serum in between each measurement (see Notes C1 and C2).
- Attach a sampler tip to the sampler. Use the sampler to draw 20 μl of the serum sample.
- Wipe the outside of the sampler tip dry with a Kimwipe, including the tip perimeter. Blot excess sample so that the meniscus is in line with the tip or slightly concave (see Note C3).
- Insert the sampler into the cooling chamber. The measurement will take approximately 1 min.
- When the measurement has been completed, pull out the sampler, and remove and discard the sampler tip.
- Dry the sampler plunger wire with a Kimwipe.
- Clean the cooling chamber with a chamber cleaner as per the manufacturer’s instructions.
- Repeat steps C5 a-f two more times (see Note C5).
- Attach a sampler tip to the sampler. Use the sampler to draw 20 μl of the serum sample.
- When all measurements have been completed, clean the cooling chamber as per the manufacturer’s guidelines.
- Report the final value as an average of all serum osmolality measurements.
- Make sure that your osmometer has been recently calibrated following the manufacturer’s guidelines, and is in good working order.
Notes
- Cardiac puncture
- Any blood collection method is sufficient for the purpose of measuring serum osmolality. We outline the cardiac puncture method as described in the original article (Stare et al., 2015).
- Since the cardiac puncture is a terminal exsanguination procedure, maintaining body heat is unnecessary. Isoflurane can be maintained at maximum levels (5%) throughout the entire procedure.
- Approximately 40-70% of the needle will need to be inserted, depending on the size of the rat. If the needle is completely inserted and there is no indication of blood in the syringe, then it is likely that the heart has been missed. In this case, withdraw the needle without removing it from the chest cavity, and try again. If an additional attempt is unsuccessful, replace the needle with a new one. Sometimes an attempt is unsuccessful because the needle becomes blocked.
- A 100-300 g rat will provide > 5 ml of blood, however only ~0.15 ml of blood is required to produce the minimum amount of serum (3 x 20 μl, see Note C1) necessary for osmolality measurement. This protocol describes the collection of 1.5 ml of blood because it is the amount that we usually collect for our own experiments (Stare et al., 2015).
- Hemolysis is the rupture of erythrocytes and can be visually detected in the serum by a change of colour from yellow/white to red. Hemolysis can alter the composition of the serum and thus potentially alter serum osmolality measurements (Lippi et al., 2012).
- A difference between serum and plasma is that serum does not contain coagulants. Therefore it is important to allow the blood to coagulate fully prior to centrifuging the sample. Storage time and temperature recommendations prior to centrifugation of the whole blood sample vary and is to this day debated (Abbadi et al., 2014; Curia et al., 2009; Seifarth et al., 2004), however we routinely place the sample on ice in an insulated foam container with the lid closed for up to 9 h without any adverse effects (see Representative data). Once the serum is isolated, it can be stored at 4 °C for up to 24 to 48 h (see Representative data; Curia et al., 2009).
- Any blood collection method is sufficient for the purpose of measuring serum osmolality. We outline the cardiac puncture method as described in the original article (Stare et al., 2015).
- Serum separation
- If the serum layer contains particles that are visible to the eye, re-centrifuge the sample at 6,000 x g for 2-5 min, and collect the serum into a fresh microcentrifuge tube.
- Occasionally a thickened, off-white substance appears in the serum layer after the blood has been centrifuged. There are two options to consider: either re-centrifuge at 6,000 x g for 2.5-5 min, or collect the serum around it if possible.
- If the serum layer contains particles that are visible to the eye, re-centrifuge the sample at 6,000 x g for 2-5 min, and collect the serum into a fresh microcentrifuge tube.
- Osmolality measurement
- The listed osmometer uses 20 μl per measurement, and has a resolution of ± 1 mOsm/kg. However, we keep a minimum of 200 μl of serum sample.
- Keep the lid of the microcentrifuge tube closed in between sampling to prevent evaporation.
- When using the sampler, make sure to closely follow the manufacturer’s guidelines to acquiring a reliable and measurable 20 μl sample. This will include ensuring that there are no bubbles in the sample, and keeping a consistent meniscus level. Consistency is very important as these subtle differences can cause inconsistencies with the measurements that may mimic calibration problems with the osmometer or sampler plunger wire deterioration.
- Should the measurements of the control solution be outside of the stated value of the standard, re-calibrate the osmometer.
- If the measured serum osmolality values are consistently varying by more than 3 mOsm/kg per test, clean the freezing chamber with alcohol as per the manufacturer’s guidelines. If values are still highly variable, replace the plunger wire in the sampler following the manufacturer’s guidelines.
- The listed osmometer uses 20 μl per measurement, and has a resolution of ± 1 mOsm/kg. However, we keep a minimum of 200 μl of serum sample.
Representative data
We investigated the effects of different coagulation times and temperatures on serum osmolality measurements determined immediately and after one day of cold storage. We found that there was no appreciable impact of various coagulation times for blood samples left at room temperature (RT, ~22 °C; 30 min and 60 min) or ice (~0 °C; 30 min, 60 min, 90 min, and 9 h; Figure 2A and 2B). We also found that serum samples were stable for a day at 4 °C (Figure 2C).
- Experimental design
Seven adult male Westar rats were subjected to the cardiac puncture procedure outlined above. Collected blood was divided into six 1.5 ml microcentrifuge tubes, and left to coagulate at RT or on ice in an insulated foam container with the lid closed for various lengths of time. Each serum sample was separated as described above, and aliquoted into 2 microcentrifuge tubes: one tube was used for immediate osmolality measurement (time point '0 h', and the other was kept at 4 °C for one day (time point '24 h'). Reported values are means of 3-7 measures per sample with ± SEM, and standard deviation (SD) where specified. Statistical analysis and graphing was performed using SigmaPlot 12 and Prism 6 to compare means by One Way ANOVA and paired t-test. A P value of < 0.05 was considered to be significant. - Results
Table 1. Sample data used for graphing the standard curve
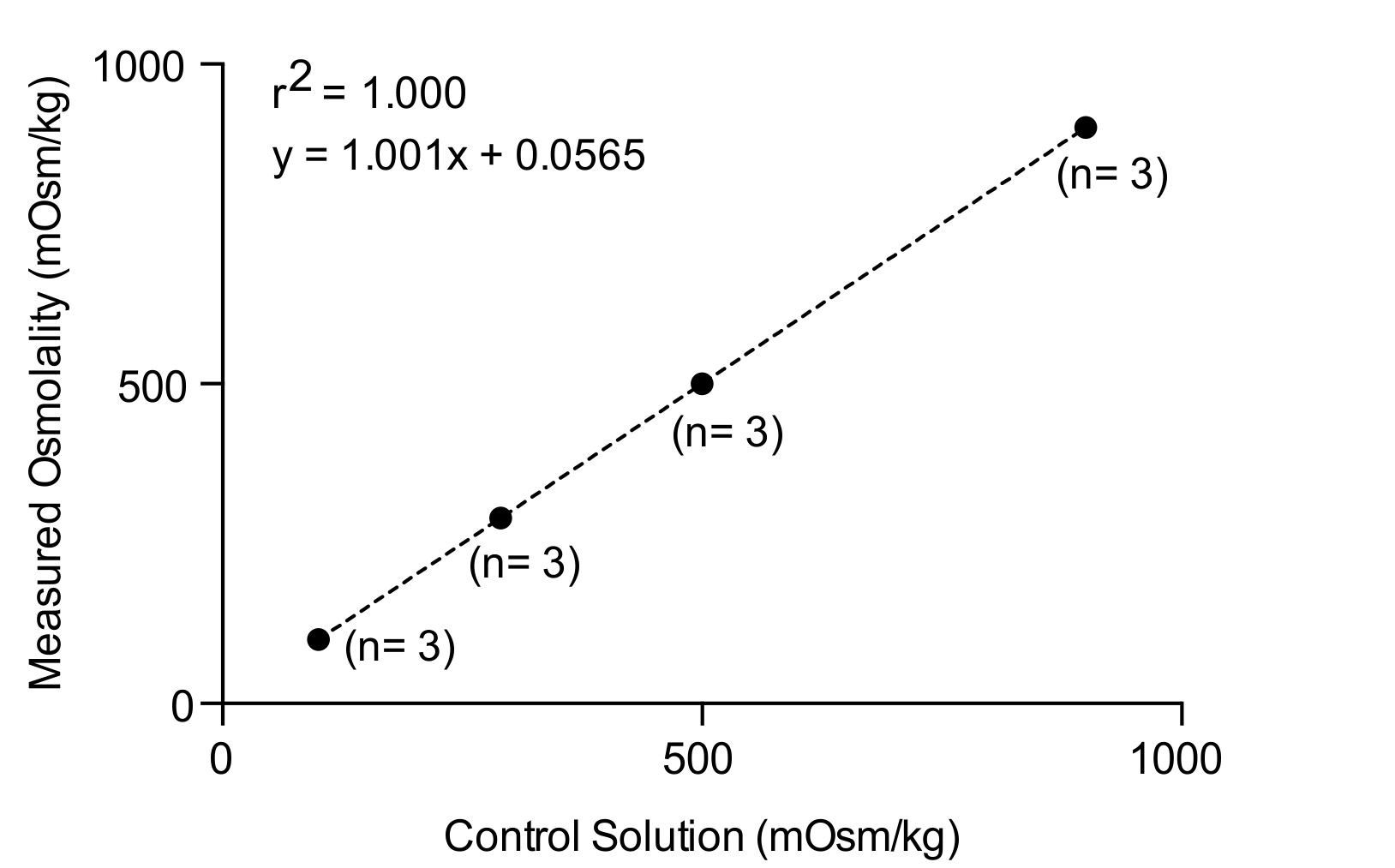
Figure 1. Standard curve used to test the calibration and performance of the osmometer used in this study
Table 2. Sample data
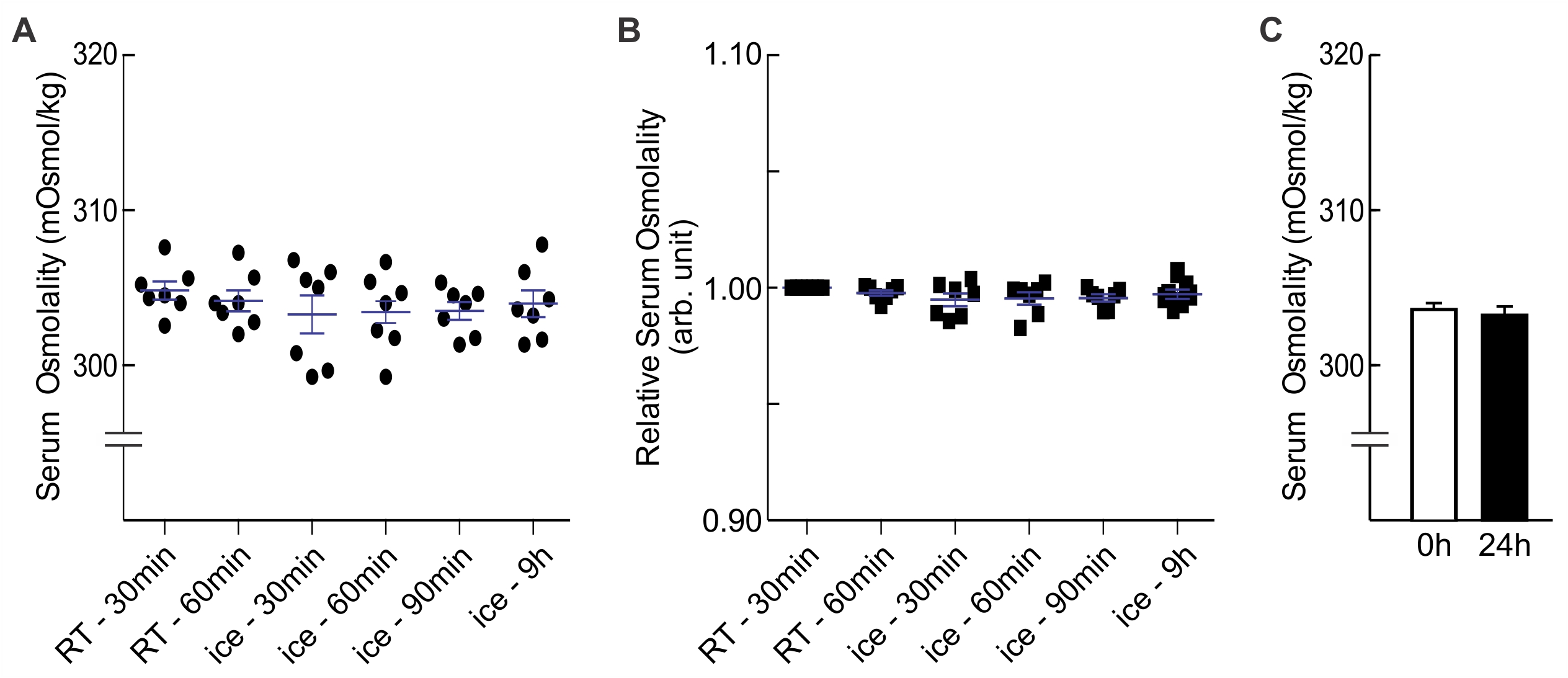
Figure 2. Effects of storage temperature and duration on serum osmolality. A. Serum osmolality values of blood samples (n = 7 each) allowed to coagulate for 30 min to 9 h at RT (~22 °C) or on ice (~0 °C). There was no statistical difference between categories (One Way ANOVA, F(5, 36) = 0.472, P = 0.795); B. Osmolality values normalized to RT-30min; C. Effect of one day of cold storage (4 °C) on serum osmolality values. There was no statistical difference (paired t-test, P = 0.241) between samples that were measured immediately and samples that were stored at 4 °C for one day (0 h, 303.72 ± 0.44 mOsm/kg, n = 26; 24 h, 303.146 ± 0.60 mOsm/kg, n = 26).
Acknowledgments
This protocol is an expanded version of the Materials and Methods – Serum Osmolality protocol appearing in Stare et al. (2015). It has been adapted from McGill Standard Operating Procedures #111-Rat Anesthesia, #301-Rodent Euthanasia and #403-Guidelines for Blood Collection Volume and Frequency, and is approved by the Facility Animal Care Committee of McGill University. Steps C2 and C5 are based on the Ease-Eject® sampler (20 μl) user guide provided by Advanced Instruments, Inc. This work was funded by the Canadian Institutes of Health Research (CIHR) Foundation Grant FDN-143337 and James McGill Research Chair to C.W.B., and a CIHR Frederick Banting and Charles Best Canada Graduate Scholarship – Doctoral Award to J.S. The Quebec Research Fund – Health supports the Research Institute of the McGill University Health Center. The authors thank Dr. M. Prager-Khoutorsky and Dr. M. Verway for providing constructive comments on this manuscript.
References
- Abbadi, A., El-Khoury, J. M. and Wang, S. (2014). Stability of serum and plasma osmolality in common clinical laboratory storage conditions. Clin Biochem 47(7-8): 686-687.
- Bourque, C. W. (2008). Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 9(7): 519-531.
- Curria, A., Pesta, M., Garry, E., Zampa, N., Rosenman, J. and Sacks, J. (2009). Refrigerated and room temperature storage stability of serum osmolality measurements. Clin Chem 55(6): A17-A18.
- Dufour D. R. (1993) Osmometry: The rational basis for use of an underappreciated diagnostic tool. American Association for Clinical Chemistry Meeting, Advanced Instruments, Inc. NY. July 13 1993.
- Ellison D. H. (2013). Hyponatremia: SIADH. In: Loriaux L. (Ed.). Endocrine Emergencies: Recognition and Treatment. Springer, 74: 115-126.
- Lippi, G., Cervellin, G., Favaloro, E. J. and Plebani, M. (2012). Effects of in vitro hemolysis on laboratory testing. In: Sonnta, O. and Plebani, M. (Eds.). In vitro and in vivo hemolysis: An unresolved dispute in laboratory medicine: 39-44.
- Lupsa B. C. and Inzucchi S. E. (2013). Diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome. In: Loriaux L. (Ed.). Endocrine Emergencies: Recognition and Treatment. Springer, 115-126.
- Reddi A. S. (2013). Disorders of water balance: Hypernatremia. In: Reddi A. S. (Ed.). Fluid, Electrolyte and Acid-Base Disorders. Springer, 133-150.
- Seifarth, C. C., Miertschischk, J., Hahn, E. G. and Hensen, J. (2004). Measurement of serum and plasma osmolality in healthy young humans--influence of time and storage conditions. Clin Chem Lab Med 42(8): 927-932.
- Stare, J., Siami, S., Trudel, E., Prager-Khoutorsky, M., Sharshar, T. and Bourque, C. W. (2015). Effects of peritoneal sepsis on rat central osmoregulatory neurons mediating thirst and vasopressin release. J Neurosci 35(35): 12188-12197.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Stare, J. and Bourque, C. W. (2016). Measuring Rat Serum Osmolality by Freezing Point Osmometry. Bio-protocol 6(19): e1950. DOI: 10.21769/BioProtoc.1950.
-
Stare, J., Siami, S., Trudel, E., Prager-Khoutorsky, M., Sharshar, T. and Bourque, C. W. (2015). Effects of peritoneal sepsis on rat central osmoregulatory neurons mediating thirst and vasopressin release. J Neurosci 35(35): 12188-12197.
Category
Biochemistry > Other compound > Blood serum
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











