- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolating Brain Mitochondria by Differential Centrifugation
Published: Vol 6, Iss 10, May 20, 2016 DOI: 10.21769/BioProtoc.1810 Views: 18326
Reviewed by: Oneil G. BhalalaTifany DesprezHong Lok Lung

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
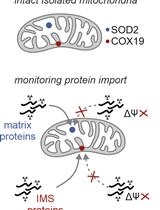
Protein Import Assay into Mitochondria Isolated from Human Cells
Lena M. Murschall [...] Jan Riemer
Jun 20, 2021 6322 Views

Isolation and Phospholipid Enrichment of Muscle Mitochondria and Mitoplasts
Alexandre Prola [...] Fanny Pilot-Storck
Oct 20, 2021 3170 Views
Analysis and Quantification of the Mitochondrial–ER Lipidome
Alexis Diaz-Vegas [...] James G. Burchfield
Jul 5, 2024 2578 Views
Abstract
In addition to methods aimed at the study of mitochondrial function in-situ, a full understanding of mitochondrial function requires their purification from cells or tissues under specific physiological or pathological conditions. This protocol illustrates a sequential procedure to obtain functional mitochondria with high yield from mice brain tissue. Mitochondria obtained with this method can be used to assess different mitochondrial parameters, including oxygen consumption, membrane potential and calcium retention capacity.
Keywords: MitochondriaMaterials and Reagents
- Centrifuge tubes
- Mice
- Sucrose (Sigma-Aldrich, catalog number: 84100 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A6003 )
- Ethylene glycol-bis(2-aminoethylether)-N, N, N’, N’-tetraacetic acid (EGTA) (Sigma-Aldrich, catalog number: E4378 )
- HEPES (Thermo Fisher Scientific, GibcoTM, catalog number: 15630-080 )
- Protease inhibitors (100x) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 78429 )
- Digitonin (Sigma-Aldrich, catalog number: D141 )
- D-Mannitol (Sigma-Aldrich, catalog number: M4125 )
- Magnesium chloride hexahydrate (MgCl2) (Sigma-Aldrich, catalog number: M9272 )
- Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: 221473 )
- Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: S5881 )
- Extraction buffer (approximately 50 ml per brain) (see Recipes)
Equipment
- Dounce homogenizer and pestles (A and B)
- Scissors
- Tweezers

Figure 1. Tools for mincing and homogenate the tissue. Dounce homogenizer and pestles (A and B), Small scissors and tweezers.
Procedure
Notes:
- To obtain a high yield of functional mitochondria the procedure must be done as fast as possible (preferably in less than 1.5 h) and the samples maintained on ice at all times.
- The procedure described here refers to mice but can be used in rats by adapting the volumes. Functional assays should be carried out within 3-4 h of isolation.
- Digitonin is added to disrupt the plasma membrane of synaptosomes and release synaptosomal mitochondria.
- Animal protocols must meet local ethics standards.
- Preparation
- Starve animals overnight (8-16 h during the dark cycle).
Note: This step is optional, but leads to more reproducible results. - Prepare a container with ice and place:
- Dounce homogenizer and pestles (Figure 1)
- Three centrifuge tubes per sample (20-50 ml)
- One beaker per sample containing cold extraction buffer
- Dounce homogenizer and pestles (Figure 1)
- Cool centrifuge and rotor to 4 °C.
- Animal dissection area.
- Small scissors (Figure 1)
- Tweezers (Figure 1)
- Small scissors (Figure 1)
- Starve animals overnight (8-16 h during the dark cycle).
- Procedure
- Sacrifice the mouse by cervical dislocation, immediately remove the complete brain and place it in the ice-cold beaker with extraction buffer.
- Rinse the brain by adding and removing cold fresh buffer until most of the blood is removed (5-6 washes) (Figure 3).
- Mince the brain in the beaker in ice extensively using small scissors (Figure 4).
- Transfer the minced brain into a Dounce homogenizer (Figure 5) and add approximately 3 ml of cold extraction buffer.
- With the homogenizer placed in the ice container, gently grind the tissue ten times with the A pestle (looser) and another ten with the B pestle (tighter). Avoiding the formation of bubbles is critical to obtain high quality mitochondria.
- Transfer the homogenate into a centrifuge tube (Figure 5) and complete to 30-40 ml with fresh cold extraction buffer. Follow the differential centrifugation steps (Figure 2).
- Centrifuge 10 min at 700 x g and 4 °C. Pour supernatant to a new ice-cold tube and discard the pellet containing nuclei and intact cells (Figure 6).
- Repeat the operation centrifuging again at 700 x g for 10 min at 4 °C and subsequently pouring the supernatant to a new ice-cold tube.
- Centrifuge at 10,000 x g for 15 min at 4 °C. Discard the supernatant and re-suspend the pellet in ice-cold extraction buffer with digitonin to a final concentration of 0.02% (Figure 7).
- Centrifuge at 10,000 x g for 15 min at 4 °C, discard the supernatant and re-suspend the final pellet in the minimal possible volume (around 0.1 ml) of extraction buffer or the specific experimental buffer (Figure 8).
Notes:- After isolation, protein concentration is determined by standard methods. Typically, around 2-3 milligrams of mitochondrial protein are obtained from one brain.
- The quality of the isolated mitochondria can be determined their respiratory control ratio (RCR) using an oxygen electrode and measuring their oxygen consumption rate in the presence and in the absence of ADP. RCR should range 4-6 with pyruvate plus malate and 1.5-3 with succinate plus rotenone.
- After isolation, protein concentration is determined by standard methods. Typically, around 2-3 milligrams of mitochondrial protein are obtained from one brain.
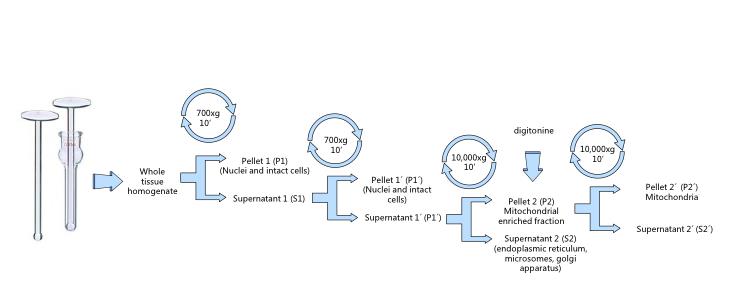
Figure 2. Mitochondrial isolation by differential centrifugation. The whole protocol must be carried at 4 °C. Avoid excessive pipetting, transfer supernatants by inversion.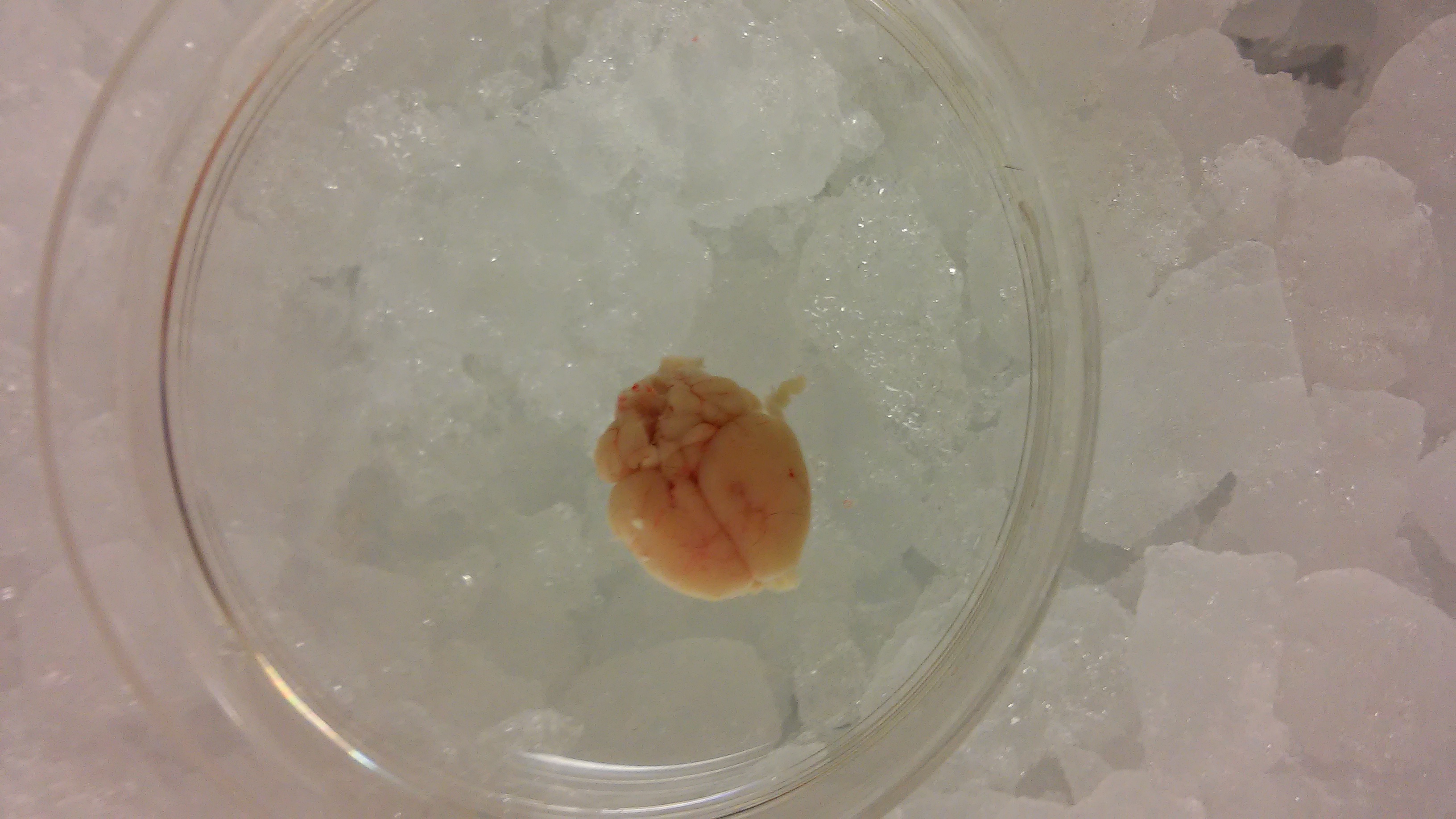
Figure 3. Extracted brain
Figure 4. Minced brain
Figure 5. Homogenized brain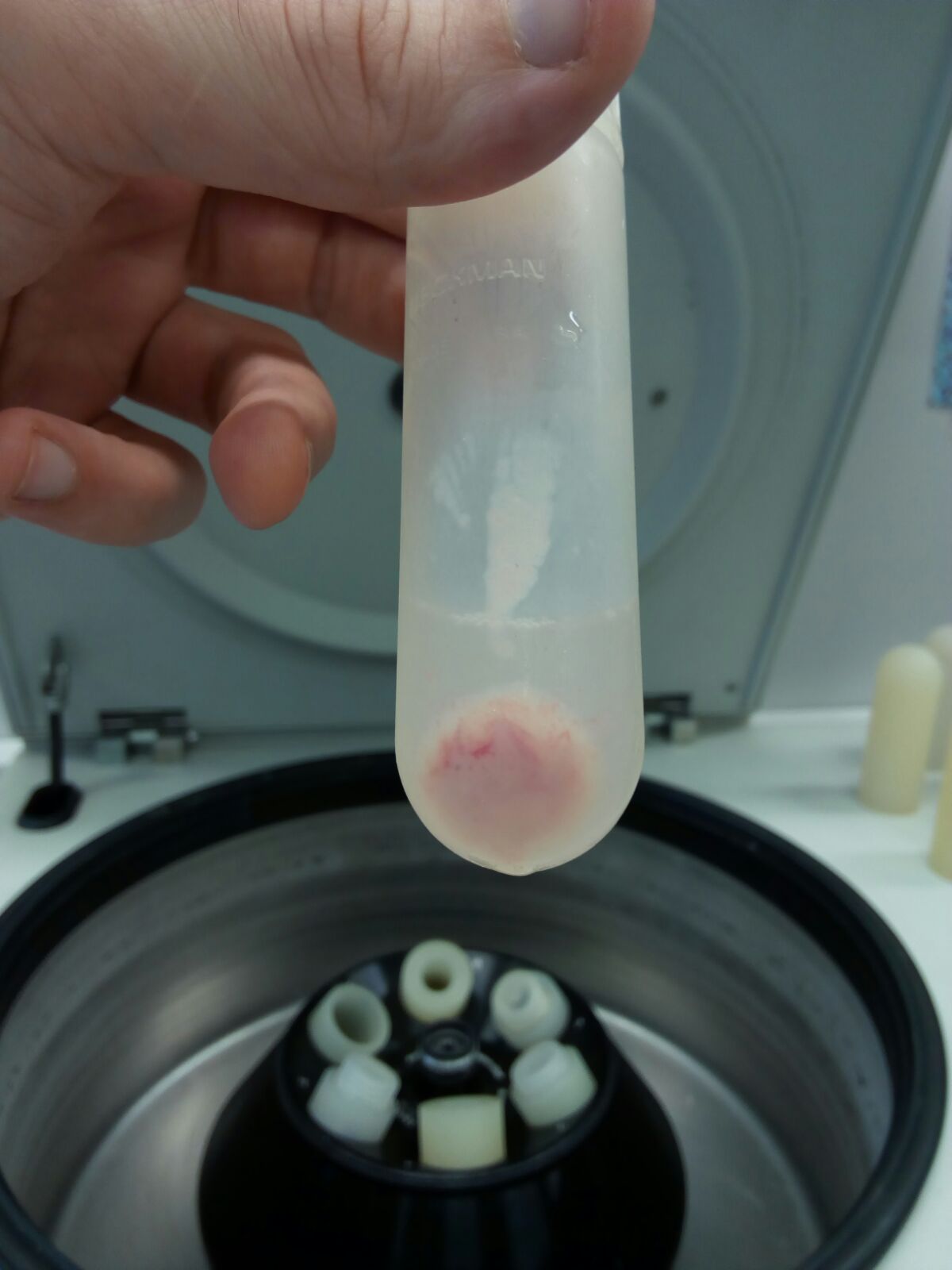
Figure 6. Pellet 1 (P1, nuclei and intact cells)
Figure 7. Pellet 2 (P2, mitochondrial enriched fraction)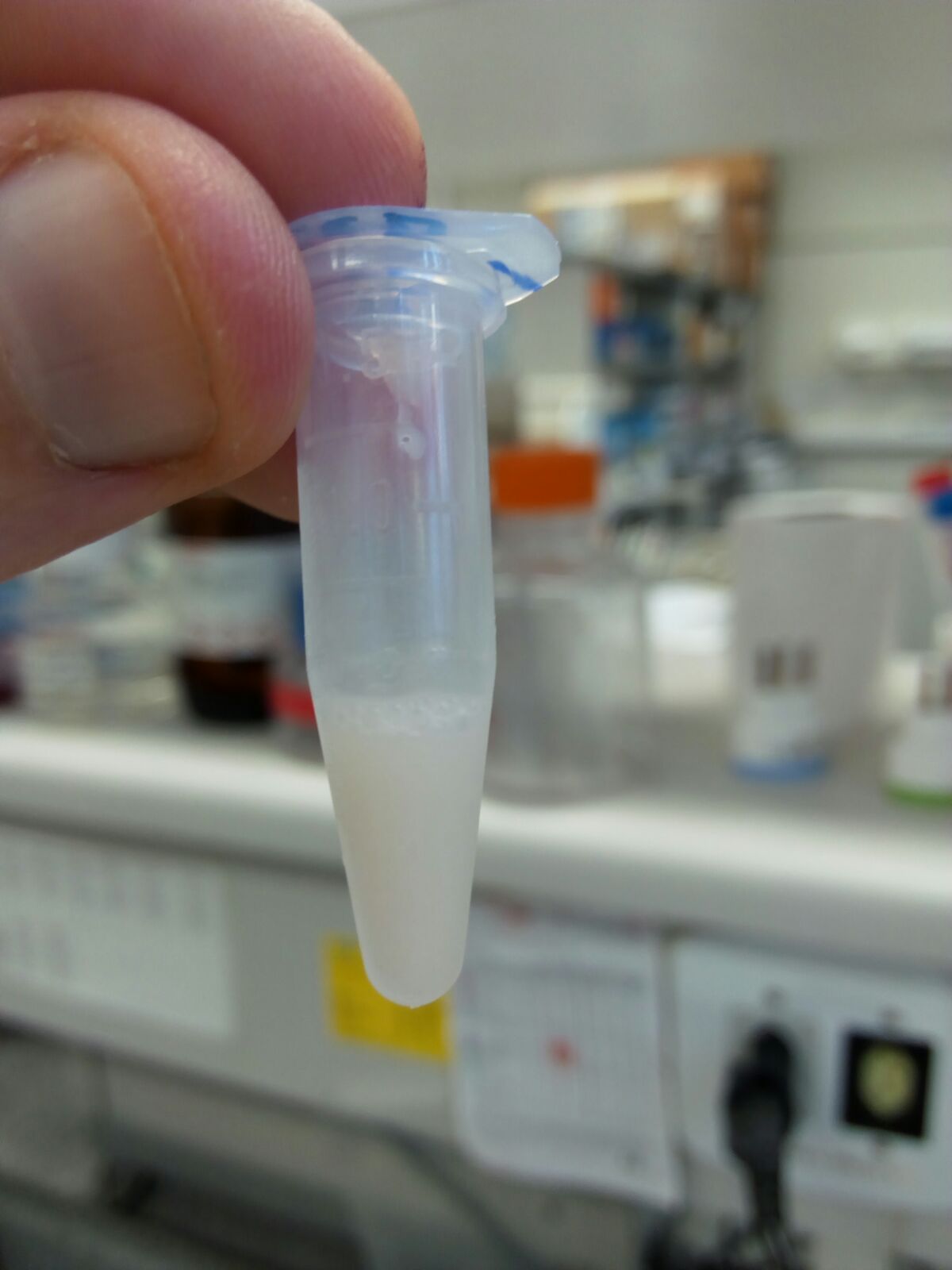
Figure 8. Re-suspended mitochondria - Sacrifice the mouse by cervical dislocation, immediately remove the complete brain and place it in the ice-cold beaker with extraction buffer.
Recipes
- Extraction buffer (freshly prepared)
125 mM sucrose
250 mM mannitol
10 mM HEPES
10 mM EGTA
0.01% BSA
1x protease inhibitors
pH 7.2 with KOH or NaOH
Note: The type of salt used can interfere with some functional assays. KOH is recommended for calcium handling experiments, as it prevents the efflux of calcium from the mitochondria through the Na+/Ca2+ exchanger. For membrane potential experiments using safranin O, NaOH is recommended to allow calibration with KOH and valinomycin.
Acknowledgments
We thank Dr. Jorgina Satrustegui, in whose laboratory our previous work was carried out, and Dr. Araceli del Arco, for constant help, guidance, support and critical comments.
References
- Rueda, C. B., Traba, J., Amigo, I., Llorente-Folch, I., Gonzalez-Sanchez, P., Pardo, B., Esteban, J. A., del Arco, A. and Satrustegui, J. (2015). Mitochondrial ATP-Mg/Pi carrier SCaMC-3/Slc25a23 counteracts PARP-1-dependent fall in mitochondrial ATP caused by excitotoxic insults in neurons. J Neurosci 35(8): 3566-3581.
- Tahara, E. B., Navarete, F. D. and Kowaltowski, A. J. (2009). Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med 46(9): 1283-1297.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Amigo, I., Traba, J. and Rueda, C. B. (2016). Isolating Brain Mitochondria by Differential Centrifugation. Bio-protocol 6(10): e1810. DOI: 10.21769/BioProtoc.1810.
-
Rueda, C. B., Traba, J., Amigo, I., Llorente-Folch, I., Gonzalez-Sanchez, P., Pardo, B., Esteban, J. A., del Arco, A. and Satrustegui, J. (2015). Mitochondrial ATP-Mg/Pi carrier SCaMC-3/Slc25a23 counteracts PARP-1-dependent fall in mitochondrial ATP caused by excitotoxic insults in neurons. J Neurosci 35(8): 3566-3581.
Category
Neuroscience > Cellular mechanisms > Mitochondria
Cell Biology > Organelle isolation > Mitochondria
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










