- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Rust Removal Experiments
Published: Vol 6, Iss 7, Apr 5, 2016 DOI: 10.21769/BioProtoc.1776 Views: 24412
Reviewed by: Valentine V TrotterModesto Redrejo-RodriguezAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
β-lactamase (Bla) Reporter-based System to Study Flagellar Type 3 Secretion in Salmonella
Fabienne F. V. Chevance and Kelly T. Hughes
Jun 20, 2023 1762 Views
Determination of Poly(3-hydroxybutyrate) Content in Cyanobacterium Synechocystis sp. PCC 6803 Using Acid Hydrolysis Followed by High-performance Liquid Chromatography
Janine Kaewbai-ngam [...] Tanakarn Monshupanee
Aug 20, 2023 1801 Views
An HPLC-based Assay to Study the Activity of Cyclic Diadenosine Monophosphate (C-di-AMP) Synthase DisA from Mycobacterium smegmatis
Avisek Mahapa [...] Dipankar Chatterji
Dec 20, 2024 1775 Views
Abstract
Iron oxidation, known as rust formation, causes enormous loss in term of property damages and associated economic risks. Depending on the degree of formation, rust consists of several layers of iron in different oxidation states. The brownish top layer is mostly iron (III) oxide-hydroxide [FeO(OH), Fe(OH)3] while the deepest black layers possess iron oxide (Fe2O3.nH2O). The flaky nature of surface rust meditates diffusion of oxygen and water to inner material sections which can lead to total disintegration of iron mass. As a result, it is desirable to remove rust and protect fresh surface from oxidizers. The common rust removal reagents are mainly based on complex formation of ferric ion with organic and inorganic acids such as citric acid, oxalic acid, and phosphoric acid. Rust removal ability is typically a qualitative observation which makes direct comparison between treatment options cumbersome if not impractical. In our recent work (Ahmadi et al., 2015), we have developed a colorimetric assay to measure ferric concentration in rust removal treatment media using a bacterially-produced siderophore (yersiniabactin) in comparison to a commercial rust removal reagent. In this approach, ferric concentration is correlated to the mass of rust being dissolved in the presence of different removal agents. This assay is based on a modification of the 1, 10-phenanthroline assay (Skoog and West, 1979) to enable detection using a 96-well plate format for higher throughput screening and assessment.
Materials and Reagents
- Rusted iron samples
- 1,10-phenanthroline (Sigma-Aldrich, catalog number: 131377 )
- Hydroxylamine hydrochloride (Sigma-Aldrich, catalog number: 159417 )
- Ammonium acetate (Sigma-Aldrich, catalog number: 09689 )
- Ammonium iron (II) sulfate hexahydrate (Sigma-Aldrich, catalog number: 09719 )
- Oxalic acid (Sigma-Aldrich, catalog number: 241172 )
- High pure water (Milli-Q)
- 0.3% 1,10-phenanthroline (wt/vol) (see Recipes)
- 1% Hydroxylamine hydrochloride (wt/vol) (see Recipes)
- 0.1 M Ammonium acetate (see Recipes)
- Ammonium iron (II) sulfate hexahydrate standard solution (see Recipes)
- 10% Oxalic acid (wt/vol) (see Recipes)
Equipment
- MaxQTM 4000 Benchtop Orbital Shakers (Thermo Fisher Scientific, catalog number: SHKA4000 )
- Biotek Synergy 4 microplate reader (http://www.biotek.com/resources/articles/hybrid-plate-reader.html)
- (Preferred) Milli-Q (Millipore) water purification system
- (Optional) Vertical Band Saw (for machine-generated iron samples)
Procedure
- Rust removal experiment
- (Optional) Machine cut the rusted iron samples (~1” x 1”).
- Clean the metal surface by washing it with acetone three times (50 ml total), rinse with excess high pure water, and air dry for 20 min.
- Insert the cleaned samples in an appropriate container (e.g., 50 ml centrifuge tube).
- Add the rust removal agent and then immediately submerge the sample in high pure water. The final volume should be recorded (V1).
- Put the tubes in shakers at 100-200 rpm.
- Collect 100 μl samples every 30 min for the desired time period (our test periods have ranged from 15 min to 2 h).
- (Optional) Machine cut the rusted iron samples (~1” x 1”).
- Remaining rust removal experiment
- Remove the metal sample and rinse it with pure water several times.
- Immerse the washed samples in a known volume (V2) of 10% (wt/vol) oxalic acid solution.
- Shake at 100-200 rpm for 30 min.
- Collect 100 μl of sample.
- Remove the metal sample and rinse it with pure water several times.
- Iron (II) calibration curve
- Add 40 μl of 1, 10-phenanthroline solution (0.3% wt/vol), 40 μl of hydroxylamine hydrochloride solution (1% wt/vol), and 40 μl of ammonium acetate (0.1 M) to each well of a 96 well plate.
- Add the iron (II) working solution: 0, 10, 20, 30, 40, 50, and 90 μl to wells containing the above mixture and adjust the final volume to 250 μl with high pure water [the iron(II) concentration will be 0, 1, 2, 3, 4, 5, and 9 mg/L, respectively]. Repeat twice more to produce triplicate curves.
- Mix thoroughly via pipetting up and down 3 times. Allow the solutions to stand for 20 min to fully develop color.
- Measure the absorbance at 510 nm.
- Plot the calibration diagram and fit the best linear curve (Figure 1).
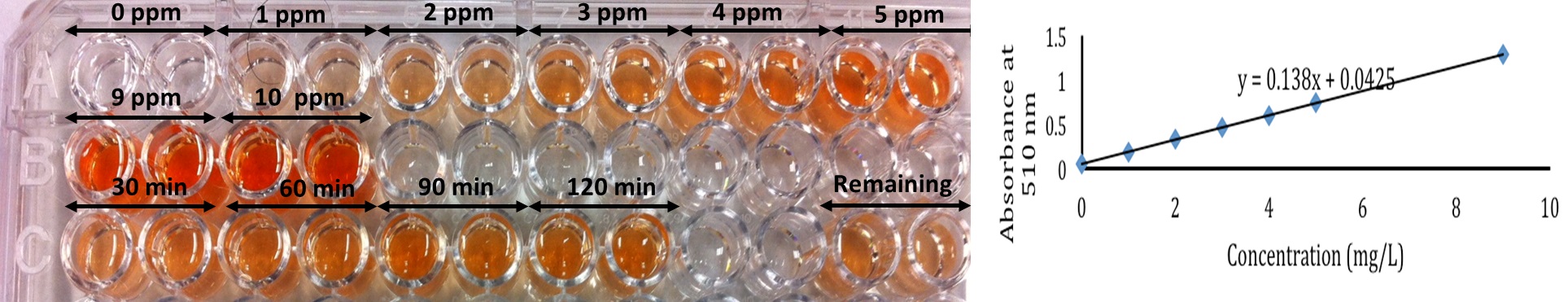
Figure 1. The developed colorimetric assay. Increasing concentration of Fe (II) from zero to 10 ppm (row A and B) increases the absorbance at 510 nm linearly. Row C includes treatment samples at different time points and remaining rust after treatment with oxalic acid.
- Add 40 μl of 1, 10-phenanthroline solution (0.3% wt/vol), 40 μl of hydroxylamine hydrochloride solution (1% wt/vol), and 40 μl of ammonium acetate (0.1 M) to each well of a 96 well plate.
- Ferric content in rust removal treatment and total rust
- Dilute the collected samples to obtain iron ion in a range of 1 to 10 ppm or alternatively add serially diluted samples. We usually dilute our metal samples 100-400 times. Record the dilution factor (D1 for samples and D2 for total rust).
- Add 20 μl of diluted samples to the 96 well plate mixture of 1,10-phenanthroline solution (40 μl of 0.3% wt/vol), hydroxylamine hydrochloride solution (40 μl of 1% wt/vol), and ammonium acetate (40 μl of 0.1 M). Adjust the volume to 250 μl per well with high pure water.
- Mix thoroughly via pipetting up and down 3 times. Allow the solutions to stand for 20 min to fully develop color.
- Measure the absorbance at 510 nm.
- Use the calibration curve to calculate the concentration (C1 for samples and C2 for total rust). Consider the dilution of samples in the total well volume
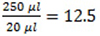 times.
times.
- Dilute the collected samples to obtain iron ion in a range of 1 to 10 ppm or alternatively add serially diluted samples. We usually dilute our metal samples 100-400 times. Record the dilution factor (D1 for samples and D2 for total rust).
- Calculation
- Removed rust (mg):
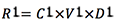
- Remaining rust (mg):
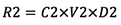
- Rust removal (%):
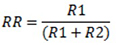
- Removed rust (mg):
Representative data
The following are typical data for a commercial rust remover (Rust-Oleum):
Table 1. Protocol results for a commercial rust remover (Rust-Oleum)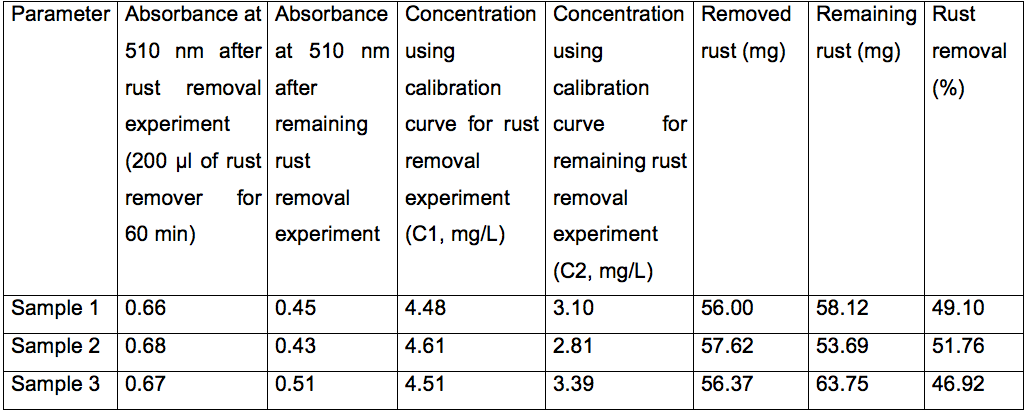
Dilution factor (D1 and D2): 1,250 times; Treatment volume for rust removal experiment (V1): 10 ml; Treatment volume for remaining rust removal experiment (V2): 15 ml.
Figure 2. Visual examples of metal samples treated with rust removal agents. Metal samples before treatment (A), after 2 h of treatment with a commercial rust removal agent (B; Rust-Oleum) and after thirty minutes treatment with 10% oxalic acid (C).
Notes
- A rusted iron sample was obtained from the University at Buffalo School of Engineering and Applied Sciences machine shop. Alternatively, rusted carbon steel can be prepared by spraying samples with acid (i.e., 100 mM HCl) and allowing subsequent air exposure for three hours.
- Instead of using oxalic acid solution (10% wt/vol), it is possible to use Clark’s solution to remove rust from steel. The solution is prepared as 2% Sb2O3 (wt/vol) and 5% SnCl2 (wt/vol) in concentrated HCl (100 ml) at room temperature with constant stirring (Moller et al., 2006). However, we have found using oxalic acid solution to be more convenient and equally suitable.
Recipes
- 0.3% 1,10-phenanthroline (wt/vol)
Dissolve 30 mg in minimum amount of methanol (~200 µl) and adjust the volume to 10 ml with high pure water - 1% Hydroxylamine hydrochloride (wt/vol)
Dissolve 100 mg in 10 ml of high pure water - 0.1 M Ammonium acetate
Dissolve 77.1 mg in 10 ml of high pure water - Ammonium iron (II) sulfate hexahydrate standard solution
Dissolve 176 mg in 10 ml of high pure water. This will serve as an iron stock solution [2,500 mg/L of iron (II)]. Dilute the stock solution 100x to make a working iron solution [25 mg/L of pure iron (II)]. - 10% Oxalic acid (wt/vol)
Dissolve 10 g of acid in 100 ml of high pure water.
Note:In all experiments high pure water has been used and unless otherwise stated, the experiments were performed at ambient temperature (~22 °C).
Acknowledgments
The authors recognize support from the New York State Pollution Prevention Institute, the NSFI-Corps program, and a SUNY-4F grant. The protocol provided here was adapted from work previously published (Ahmadi et al., 2015).
References
- Ahmadi, M. K., Fawaz, S., Jones, C. H., Zhang, G. and Pfeifer, B. A. (2015). Total biosynthesis and diverse applications of the nonribosomal peptide-polyketide siderophore yersiniabactin. Appl Environ Microbiol 81(16): 5290-5298.
- Moller, H., Boshoff, E. T. and Froneman, H. (2006). The corrosion behaviour of a low carbon steel in natural and synthetic seawaters. Journal of the South African Institute of Mining and Metallurgy 106(8): 585-592.
- Skoog, D. A. and West, D. M. (1979). Fundamentals of analytical chemistry, 2nd ed. Mir. Chapter 29.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ahmadi, M. K. and Pfeifer, B. A. (2016). Rust Removal Experiments. Bio-protocol 6(7): e1776. DOI: 10.21769/BioProtoc.1776.
Category
Microbiology > Microbial biochemistry > Other compound
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










