- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2014
Volume: 4, Issue: 11
Biochemistry
Small-scale Triton X-114 Extraction of Hydrophobic Proteins
Protocol for Preparation of Nuclear Protein from Mouse Lungs
Determination of Pseudokinase-ligand Interaction by a Fluorescence-based Thermal Shift Assay
Cell Biology
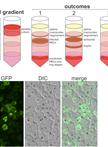
Small-scale Subcellular Fractionation with Sucrose Step Gradient
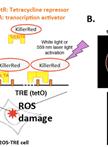
Novel Method for Site-specific Induction of Oxidative DNA Damage to Study Recruitment of Repair Proteins to Heterochromatin and Euchromatin
Immunology
Protocol for Macrophage Depletion from Mice
In vitro Inflammasome Assay
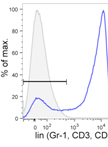
Identification of Helminth-induced Type 2 CD4+ T Cells and ILC2s
Microbiology
Preparation of Parasite Protein Extracts and Western Blot Analysis
Intracellular Glycogen Assays
Infant Rabbit Colonization Competition Assays
Immuno-EM Analysis of PF13_0191-GFP Expressing Parasites
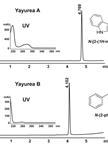
Purification and Structural Analysis of QS-inhibiting Compounds from Staphylococcus delphini
Stem Cell
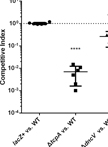
Competitive Bone-marrow Transplantations