- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2016
Volume: 6, Issue: 19
Biochemistry
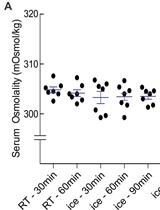
Measuring Rat Serum Osmolality by Freezing Point Osmometry
Measurement of Glucose-6-phosphate Dehydrogenase Activity in Bacterial Cell-free Extracts
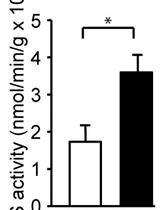
Spectrophotometric Determination of Glutamine Synthetase Activity in Cultured Cells
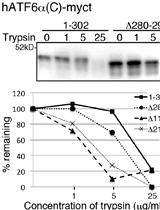
Trypsin Sensitivity Assay to Study the Folding Status of Proteins
Cell Wall-bound p-Coumaric and Ferulic Acid Analysis
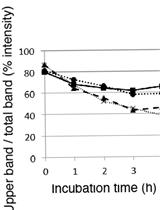
PNGase Sensitivity Assay to Study the Folding Status of Proteins
Cancer Biology
Evaluation of Angiogenesis Inhibitors Using the HUVEC Fibrin Bead Sprouting Assay
Isolation of Primary Breast Cancer Cells from HER2 Transgenic Mice
Cell Biology
Detection of Reactive Oxygen Species Using MitoSOX and CellROX in Zebrafish
Mouse Corneal Stroma Fibroblast Primary Cell Culture
Developmental Biology
Isolation, Culture, and Staining of Single Myofibers
Immunology
In vivo Analysis of Neutrophil Infiltration during LPS-induced Peritonitis
ASC-particle-induced Peritonitis
Microbiology
Measurement of Cellular Copper in Rhodobacter capsulatus by Atomic Absorption Spectroscopy
Molecular Biology
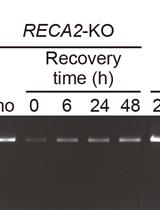
PCR-based Assay for Genome Integrity after Methyl Methanesulfonate Damage in Physcomitrella patens
Neuroscience
Sucrose Preference Test to Measure Anhedonic Behaviour in Mice
Light/Dark Transition Test to Assess Anxiety-like Behavior in Mice
Organotypic Spinal Cord Slice Cultures and a Method to Detect Cell Proliferation in These Slices
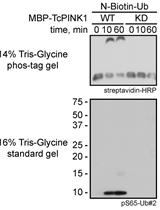
Non-radioactive in vitro PINK1 Kinase Assays Using Ubiquitin or Parkin as Substrate
Plant Science
An Assay to Study Botrytis cinerea-infected Grapevine Leaves Primed with Pseudomonas fluorescens