Dissection and Staining of Mouse Brain Ventricular Wall for the Analysis of Ependymal Cell Cilia Organization
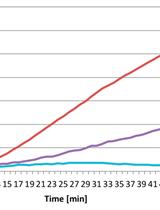
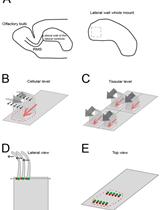
In the developing and mature central nervous system (CNS) the ventricular lumen is lined by the neuroepithelium and ependymal, respectively. These ventricular epithelia perform important functions related to the development, morphogenesis and physiology of the brain. In the mature CNS, ependyma constitutes a barrier between brain parenchyma and cerebro- spinal fluid (CSF). The most prominent feature of the apical surface of ependymal cells is the presence of multiple motile cilia that extend towards the ventricular lumen. The beating of cilia ensures the circulation of the CSF and its impairment leads to hydrocephalus. For an effective CSF flow, ciliary beating must be coordinated at the level of individual cells and at the tissue level. This coordination is achieved through the precise organization of cilia positioning within the plane of the ependyma. Two major features have been described regarding the planar organization of cilia in ependymal cells (Mirzadeh et al., 2010) and both have a cellular and tissular aspect (Boutin et al., 2014). The first one, rotational polarity, refers to the orientation of ciliary beating. At the cellular level, all cilia beat in the same direction (Figure 1B, black arrows). At the tissue level, each ependymal cell coordinates the direction of their beating with that of neighboring cells (Figure 1C, grey arrows). The second feature, translational polarity, is unique to ependymal cells and refers to the clustering of cilia in a tuft. At the cellular level, this tuft is displaced relative to the center of the ependymal cell (Figure 1B, red arrow). At the tissue level, the positioning of the ciliary tuft is coordinated between adjacent cells (Figure 1C). Alteration of any of these polarities at either level impairs CSF flow circulation (Mirzadeh et al., 2010; Boutin et al., 2014; Guirao et al., 2010; Hirota et al., 2010; Ohata et al., 2014). Cilia axonemes arise from basal bodies (BB) which are cylindrical structures anchored perpendicular to the sub-apical surface of the cells (Figure 1D). BBs are polarized by the presence of appendices such as basal foot or striated rootlets. The basal foot protrudes in a direction correlated with the direction of cilia beating, while the striated rootlet protrudes in the opposite direction of cilia beating (Marshall, 2008). The ‘en face view’ observation of BBs’ organization allows the visualization of ependymal polarities (Mirzadeh et al., 2010; Boutin et al., 2014). Here, we describe an immunofluorescence (IF) protocol for observation of ciliated cells in mouse brain ventricular lateral wall whole mounts (LWWM). This protocol can be used for classical confocal microscopy analysis. In addition, it is well suited for super-resolution STimulated Emission Depletion (STED) microscopy if observation of structures that have features which are smaller than the optical diffraction limit is needed. Finally, we describe a combination of antibodies that allow the concomitant observation, in a single sample, of ependymal polarities at the level of individual cilia, individual cells and at the tissue level.