- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2015
Volume: 5, Issue: 24
Cancer Biology
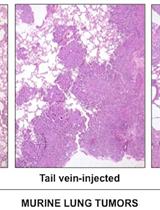
Analysis of Murine Lung Tumors by Micro PET-CT Imaging
Generation of Mouse Thyroid Calcitonin-producing Cell Tumors from Primary Mouse Tumors
Microbiology
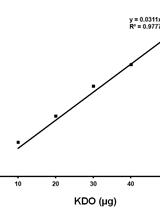
Determination of Keto-deoxy-d-manno-8-octanoic acid (KDO) from Lipopolysaccharide of Escherichia coli
Preparation and Analysis of Crude Autolytic Enzyme Extracts from Staphylococcus aureus
Transformation of the Cyanobacterium Leptolyngbya boryana by Electroporation
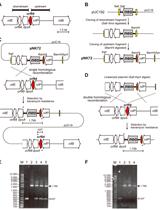
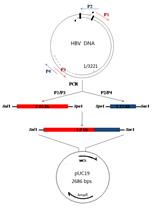
Characterization of HBV Isolates from Patient Serum Samples and Cloning
Extraction and Quantification of Alkanes in Cyanobacteria
[14C] Linoleic Acid Uptake and Fractionation Assay in Vibrio cholerae
Plant Science
Luminol-based Assay for Detection of Immunity Elicitor-induced Hydrogen Peroxide Production in Arabidopsis thaliana Leaves
Extraction of Apoplastic Wash Fluids and Leaf Petiole Exudates from Leaves of Arabidopsis thaliana
GC-MS-Based Analysis of Chloroform Extracted Suberin-Associated Root Waxes from Arabidopsis and Other Plant Species
In vitro CLE Peptide Bioactivity Assay on Plant Roots
Isolation of Tonoplast Vesicles from Tomato Fruit Pericarp
Insertional Mutagenesis of Chlamydomonas reinhardtii
Stem Cell
Porous Scaffold Seeding and Chondrogenic Differentiation of BMSC-seeded Scaffolds













![[14C] Linoleic Acid Uptake and Fractionation Assay in Vibrio cholerae](https://en-cdn.bio-protocol.org/imageup/arcimg/20151221041353472.jpg?t=1766119334)