- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2024
Volume: 14, Issue: 10
Cell Biology
A Standardized Protocol for Extraction and Homogenization of Ocular Tissues
Reversible Photoregulation of Cell–Cell Adhesions With Opto-E-cadherin
Developmental Biology
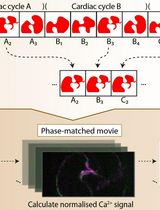
Calcium Signal Analysis in the Zebrafish Heart via Phase Matching of the Cardiac Cycle
Medicine
A Detailed Protocol for the Induction of Anemia and RBC Transfusion–associated Necrotizing Enterocolitis in Neonatal Mice
Molecular Biology
Fluorescent Labeling and Imaging of IL-22 mRNA-Loaded Lipid Nanoparticles
Neuroscience
Optogenetic Interrogation of Electrophysiological Dendritic Properties and Their Effect on Pacemaking Neurons from Acute Rodent Brain Slices
Plant Science
Simplified Protocol to Demonstrate Gene Expression in Nicotiana benthamiana Using an Agrobacterium-Mediated Transient Assay