- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2023
Volume: 13, Issue: 12
Biochemistry
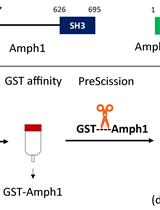
Purification of Recombinant Human Amphiphysin 1 and its N-BAR Domain
Cell Biology
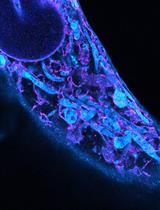
Ten-fold Robust Expansion Microscopy
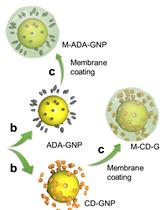
Synthesis of Bacteria-mimetic Gold Nanoparticles for Phagocytosis by Immune Cells
Environmental science
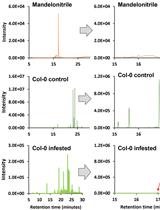
Isolation and Quantification of Mandelonitrile from Arabidopsis thaliana Using Gas Chromatography/Mass Spectrometry
Immunology
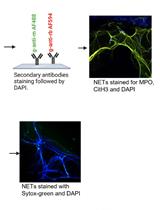
A New Methodology for the Quantification of Neutrophil Extracellular Traps in Patient Plasma
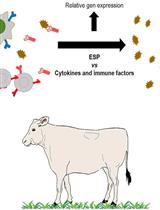
Selection of Molecules with Immunological Potential from Excretory and Secretory Products from the Nematode Haemonchus placei by Cell Proliferation and Gene Expression Assays
Microbiology
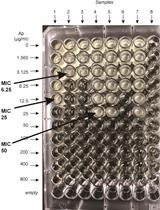
β-lactamase (Bla) Reporter-based System to Study Flagellar Type 3 Secretion in Salmonella
Neuroscience
Alginate Gel Immobilization of Caenorhabditis elegans for Optical Calcium Imaging of Neurons
A Standardized Protocol for Early-life Stress-induced Social Defeat in Mice
Triplet-primed PCR and Melting Curve Analysis for Rapid Molecular Screening of Spinocerebellar Ataxia Types 1, 2, and 3