- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2023
Volume: 13, Issue: 7
Biochemistry
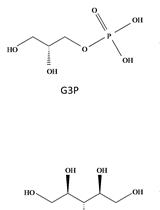
Rapid and Reliable Quantification of Glycerol-3-phosphate Using Gas Chromatography–coupled Mass Spectrometry
Biological Engineering
Preparation and Characterization of IL-22 mRNA-Loaded Lipid Nanoparticles
Cancer Biology
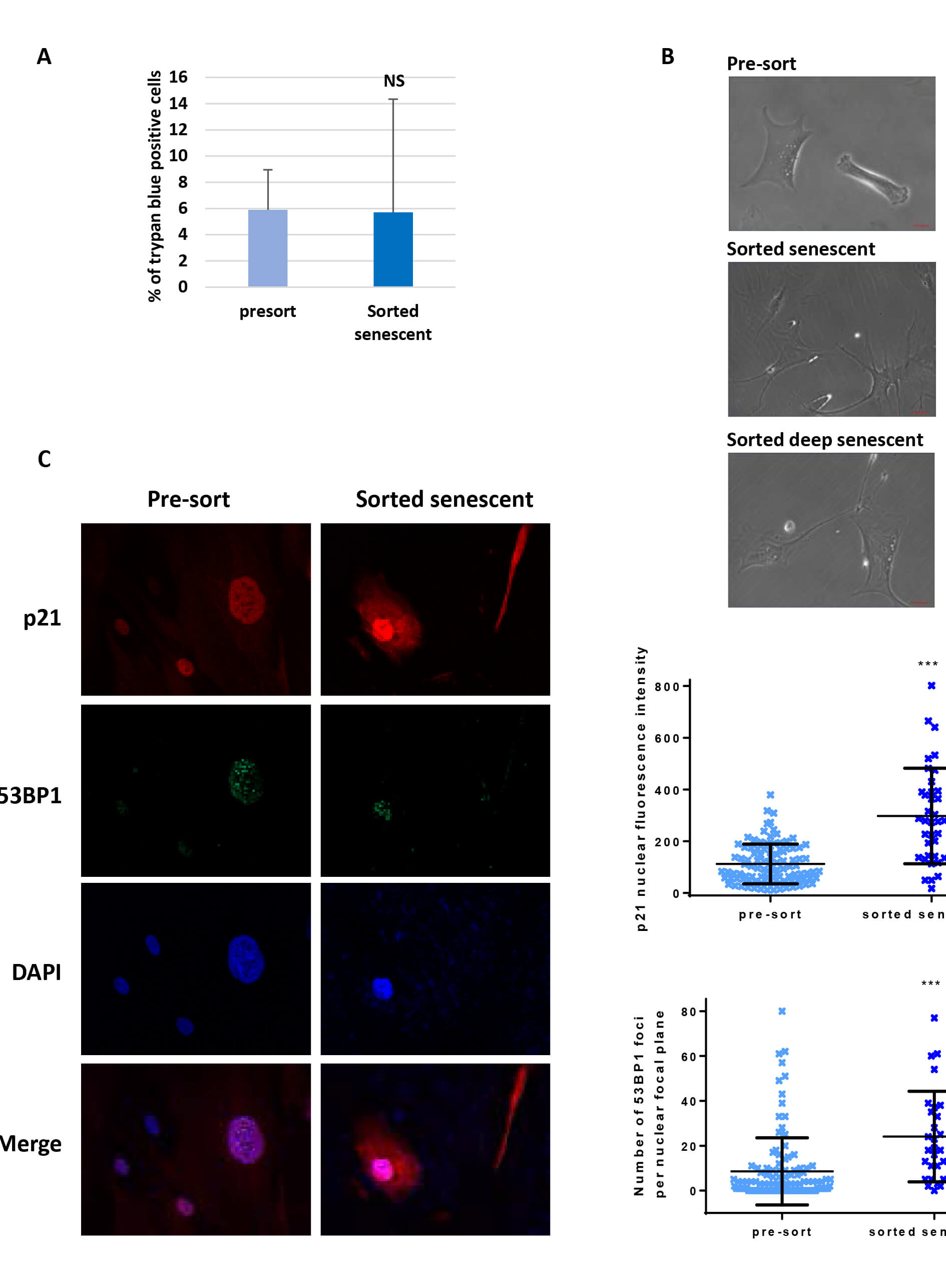
Flow Cytometry-based Method for Efficient Sorting of Senescent Cells
Developmental Biology
Analysis of Mouse Brain Sections by Live-cell Time-lapse Confocal Microscopy
Immunology
In situ Microinflammation Detection Using Gold Nanoclusters and a Tissue-clearing Method
Neuroscience
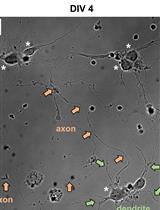
Fluorescence Assays for Real-Time Tracking of Cell Surface Protein Internalization and Endosomal Sorting in Axons of Primary Mouse Hippocampal Neurons
Reconstitution of Membrane-tethered Postsynaptic Density Condensates Using Supported Lipid Bilayer
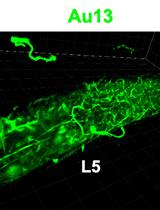
Isolation of Immunocomplexes from Zebrafish Brain
Plant Science
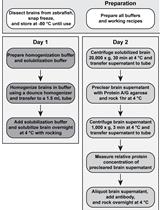
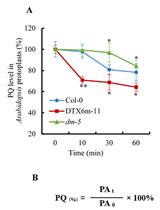
Determination of Paraquat in Arabidopsis Tissues and Protoplasts by UHPLC-MS/MS