- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2022
Volume: 12, Issue: 2
Biological Engineering
Measuring Oligonucleotide Hydrolysis in Cellular Lysates via Viscosity Measurements
Cancer Biology
Preparation and Cultivation of Colonic and Small Intestinal Murine Organoids Including Analysis of Gene Expression and Organoid Viability
An Alternative Technique for Monitoring the Live Interaction of Monocytes and Tumor Cells with Nanoparticles in the Mouse Lung
Developmental Biology
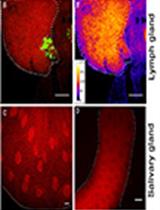
Combination of Immunofluorescence and Quantitative Fluorescence In-situ Hybridization for Analysing Differential Gene Expression in the Niche Cells of the Drosophila Lymph Gland
Drug Discovery
Rapid in vitro and in vivo Evaluation of Antimicrobial Formulations Using Bioluminescent Pathogenic Bacteria
Immunology
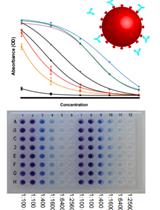
A High-throughput Automated ELISA Assay for Detection of IgG Antibodies to the SARS-CoV-2 Spike Protein
Microbiology
Quantification of Bacterial Loads in Caenorhabditis elegans
Simple Scalable Protein Expression and Extraction Using Two-stage Autoinducible Cell Autolysis and DNA/RNA Autohydrolysis in Escherichia coli
High-throughput Growth Measurements of Yeast Exposed to Visible Light
Molecular Biology
ATAC Sequencing Protocol For Cryopreserved Mammalian Cells
Neuroscience
Trichloroacetic Acid Fixation and Antibody Staining of Zebrafish Larvae
Reconstitution of Membrane-associated Components of a G-protein Signaling Pathway on Membrane-coated Nanoparticles (Lipobeads)
Plant Science
Fractionation and Extraction of Crude Nuclear Proteins From Arabidopsis Seedlings
Rhizoctonia solani Infection Assay of Young Sugar Beet and Arabidopsis plantlets
Stem Cell
From 3D to 2D: Harmonization of Protocols for Two-dimensional Cultures on Cell Culture Inserts of Intestinal Organoids from Various Species
Flow Cytometry Analysis of Planarian Stem Cells Using DNA and Mitochondrial Dyes