- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2019
Volume: 9, Issue: 16
Biochemistry
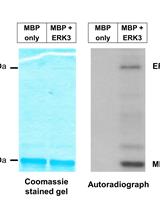
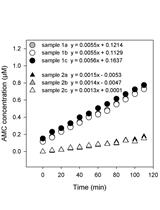
A Radioactive in vitro ERK3 Kinase Assay
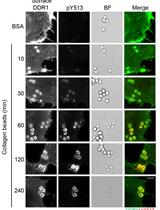
Cell-based Assay for Recruitment of DDR1 to Collagen-coated Beads
Cancer Biology
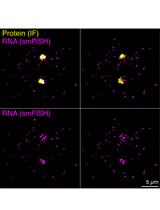
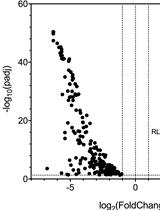
Three-dimensional Reconstruction and Quantification of Proteins and mRNAs at the Single-cell Level in Cultured Cells
Cell Biology
A Novel Technique for Imaging and Analysis of Hair Cells in the Organ of Corti Using Modified Sca/eS and Machine Learning
Fibroblast Gap-closure Assay-Microscopy-based in vitro Assay Measuring the Migration of Murine Fibroblasts
Developmental Biology
Ex vivo Drosophila Wing Imaginal Disc Culture and Furin Inhibitor Assay
Immunology
In vitro Differentiation of Thymic Treg Cell Progenitors to Mature Thymic Treg Cells
Microbiology
Non-invasive Quantification of Cell Wall Porosity by Fluorescence Quenching Microscopy
Assessment of Metacaspase Activity in Phytoplankton
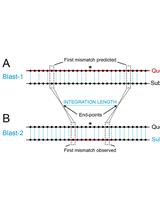
Measurement of the Length of the Integrated Donor DNA during Bacillus subtilis Natural Chromosomal Transformation
Molecular Biology
Application of a Modified Smart-seq2 Sample Preparation Protocol for Rare Cell Full-length Single-cell mRNA Sequencing to Mouse Oocytes
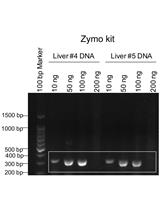
Genotyping of the OATP1B1 c. 521 T>C Polymorphism from the Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Specimens: An Optimized Protocol
Neuroscience
Cylinder Test to Assess Sensory-motor Function in a Mouse Model of Parkinson’s Disease
Construction of Viral Vectors for Cell Type-specific CRISPR Gene Editing in the Adult Mouse Brain
Whisker Nuisance Test: A Valuable Tool to Assess Tactile Hypersensitivity in Mice