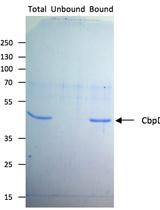
Isolation of Chloroplast Inner and Outer Envelope Membranes
The chloroplast is an important organelle found in plant cells that conduct photosynthesis. It is enclosed by a pair of closely spaced membranes, the double-membrane envelope, consisting of the inner membrane bounding the matrix or stroma and the outer membrane in contact with the cytoplasm. Like many bio-membranes, the chloroplast envelope plays an important role in mediating the complex interactions between the chloroplast and the cytoplasm. The envelope is also the site of various biosynthetic reactions, including the formation of the galactolipids, which are the major components of both envelope and the thylakoid membranes. The inner and outer envelope membranes have differences in both structure and function. For example, the outer membrane exhibits lower density of intramembranous particles than the inner membrane dose, suggesting that the protein content of the outer membrane is low. Also, the outer membrane is nonspecifically permeable to low molecular weight compounds, whereas the inner is impermeable to such compounds and contains several translocator systems for the transport of metabolites. To prepare the envelope membranes, it is necessary to isolate intact chloroplasts first. Then the inner and outer envelope membranes are separated by: 1) the protease-treatment method and 2) the centrifuge method which based on the fact that the outer envelope is lighter and the inner membrane heavier. Both methods need to isolate the intact chloroplasts firstly. However, the centrifugal separation can get the pure inner and outer envelope preparations, which therefore are suitable to the subsequent analyses. Also, the centrifuge method can avoid the destruction of inner envelope polypeptides during the protease treatment, because some of the protease may gain access to the inner membrane. Moreover, the centrifuge method is easy to operate and to get the complete enveloped that contain less of the adhesion regions of the outer and inner envelope membranes. Here we describe a reliable method for isolation of the inner and outer envelope membranes of the chloroplasts from tobacco, which is the plant that relatively not easy to use for envelope isolation.