- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2015
Volume: 5, Issue: 2
Immunology
Phenotyping of Live Human PBMC using CyTOFTM Mass Cytometry
Isolation of CNS-infiltrating and Resident Microglial Cells
Microbiology
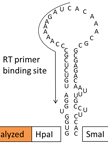
Determination of the Secondary Structure of an RNA fragment in Solution: Selective 2`-Hydroxyl Acylation Analyzed by Primer Extension Assay (SHAPE)
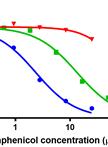
In vitro Dynamic Model of a Catheterized Bladder and Biofilm Assay
Loading of Cells with Fluorescent Probe to Study Intracellular Acid-base Homeostasis in Lactic Acid Bacteria
Mitochondrial Biogenesis Assay after 5-day Treatment in PC-3 Cells
Molecular Biology
Determination of Mitochondrial DNA Upon Drug Treatment
Plant Science
Camalexin Quantification in Arabidopsis thaliana Leaves Infected with Botrytis cinerea
Stem Cell
PBMC-MSC Co-cultures for Induction of Treg Generation
Monocyte-MSC Co-cultures