- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vivo Fluorescein Isothiocyanate-dextran (FD4) Permeability Assay
Published: Vol 5, Iss 20, Oct 20, 2015 DOI: 10.21769/BioProtoc.1618 Views: 25457
Reviewed by: Ningfei AnAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Systematic Analysis of Smooth Muscle and Cartilage Ring Formation during Mouse Tracheal Tubulogenesis
Haoyu Wu [...] Wenguang Yin
Jul 5, 2023 1534 Views

Protocol for the Implantation of Scaffolds in a Humanized Mouse Cutaneous Excisional Wound Healing Model
Dina Gadalla [...] David G. Lott
Sep 20, 2024 1451 Views

Dissection and Whole-Mount Immunofluorescent Staining of Mouse Hind Paw Muscles for Neuromuscular Junction Analysis
Rebecca L. Simkin [...] James N. Sleigh
May 20, 2025 3909 Views
Abstract
Using pluripotent stem cells, it is now becoming possible to develop tissue models of organ systems within the body. These organs will allow for the study of organ function, physiology, embryology, and even pathologic processes. Recently, our group developed a model of human small intestine developed from human pluripotent stem cells which when transplanted in vivo, produce a mature, cystic intestinal structure that has digestive functions similar to that of native small intestine (Watson et al., 2014). Intestinal permeability is a primordial function of both the epithelium and associated tight junctions to control nutrient intake and prevent the passage of pathogens. One way to study gastrointestinal paracellular permeability is by determining the ability of fluorophores-conjugated macromolecules (i.e., fluorescein isothiocyanate-dextran (FITC-dextran; or FD4) to cross from the lumen and into circulation (Dong et al., 2014). We were able to test the intestinal permeability by injecting FITC-dextran directly into the lumen of the bioengineered intestine and determining the fluorescence within the blood of the murine host at various time points after injection.
Materials and Reagents
- EDTA blood collection tubes (BD Biosciences, catalog number: 365873 )
- 1 ml syringes
- 30 gauge needles
- Microhematocrit Capillary Tubes (Thermo Fisher Scientific, catalog number: 22-362-566 )
- 1 ml Eppendorf tubes (USA Scientific, catalog number: 1615-5510 )
- 96-well microplates (Greiner Bio-One GmbH, catalog number: 655101 )
- Immune deficient NOD-SCID IL-2Rγnull (NSG) mice, 8-16 weeks of age (bred in house), transplanted with a bioengineered intestine (maturation of transplant then allowed for 6-8 weeks)
- Fluorescein isothiocyanate-conjugated dextran (FITC-dextran 3-5 Kda) (Sigma-Aldrich, catalog number: FD4 )
- Sterile water
- Phosphate-buffered saline (PBS)
- Isoflurane for inhaled anesthesia (Henry Schein, catalog number: 050033 )
- Buprenorphine (Midwest Veterinary Supply, catalog number: 790.06700.3 )
- Isopropyl alcohol
- Povidone-Iodine
- Small surgical kit (scissors, ringed forceps, needle driver/holder)
- 3-0 Vicryl suture (Ethicon US, catalog number: VR416 )
- GLUture tissue adhesive 1.5 ml 1/Tb (Skin closure glue) (Henry Schein, catalog number: 034418 )
Equipment
- Spectrophotofluorometer (BioTek Instruments, model: Synergy H1 )
- Dissecting Stereomicroscope (Leica Microsystems)
- Microcentrifuge
- Hair clipper/trimmer
Procedure
- Dissolve FITC-Dextran in sterile water at a final concentration of 20 mg/ml.
- Trim the hair over the abdomen of a transplanted NSG mouse then clean the skin with isopropyl alcohol and povidone-iodine to create a sterile field.
- Induce anesthesia via nose cone with 2% inhaled Isofluorane.
- Once the mouse is anesthetized, cut the skin of the abdomen using scissors for several centimeters over the region of the transplanted tissue.
- Bring and secure the desired transplanted intestinal tissue up into the incision field using a dissecting microscope.
- Draw 100 μl of the FITC-Dextran solution into a 1 ml syringe equipped with a 30 gauge injection needle.
- Inject the lumen of the transplant with 100 μl of FITC-Dextran solution.
- Seal the injection site with skin glue to prevent leakage of the solution into the peritoneal cavity.
- Close the skin of the mouse using 3-0 vicryl suture in a running fashion.
- Give the mice a subcutaneous injection of buprenorphine (0.05 mg/kg) for analgesia.
- Remove the mouse from anesthesia and place the mouse into a clean, warm area for recovery.
- At 30 min after injection, return the injected mouse to the anesthesia machine for inhaled isoflurane anesthesia.
- Collect blood in EDTA blood collection tubes via retro-orbital bleeding using heparinized capillary tubes. Mix by inverting the tube 3-4 times.
- Return the mouse to the recovery area until the second timepoint of 4 h from the initial injection.
- Repeat step 13 at 4 h after injection.
- Sacrifice the mice using a CO2 exposure and confirm the euthanasia by cervical dislocation and collect tissues for histology at this time if desired. All animal euthanasia must be performed in accordance with the IACUC guidelines and an approved animal protocol within the institution.
- Centrifuge the EDTA tubes at 300 rpm for 10 min at 4 °C using the microcentrifuge.
- Remove the serum supernatant from the EDTA tubes.
- Dilute each sample in an equivalent amount of chilled PBS.
- Prepare a standard curve using serial dilution of FITC-Dextran in PBS (0, 125, 250, 375, 500, 750, 1,000 µg/ml).
- Add 100 μl of diluted serum to a 96-well, flat-bottom plate in duplicates. Remember to include a negative control with serum from a mouse that was not injected with FITC-Dextran solution.
- Determine the concentration of FITC-Dextran in the serum using a spectrophotometer (Excitation: 485 nm; Emission: 528 nm).
- Plot the data to determine the concentration of the sample using the standard curve (Figure1).
Representative data
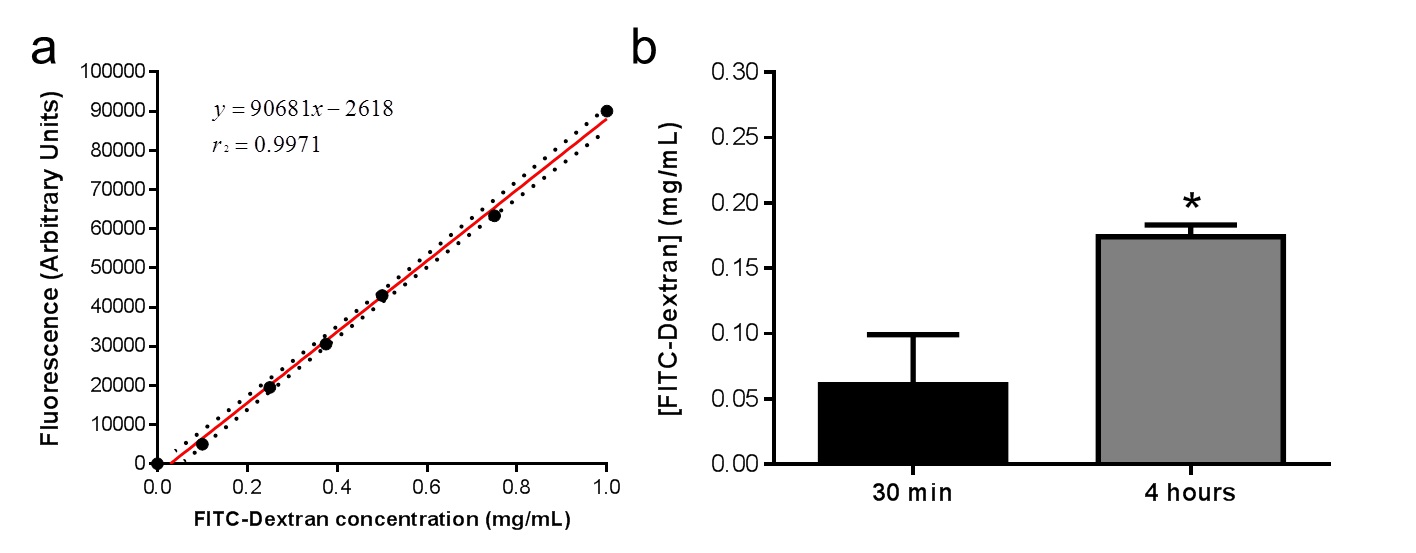
Figure 1. a. Standard curve obtained from serial dilution of FITC-Dextran in PBS. b. FITC-Dextran injected into engraftments in vivo increased significantly in murine serum from initial timepoint of 30 min compared with timepoint of 4 h within each mouse (n=7). Values represented in the graph represent Mean ± s.e.m.; *p <0.05.
Notes
- This protocol can be adapted for other experiments involving other types of transplanted tissue or even adapted for native intestinal tissues in animal models. Any hollow viscus can be injected in a similar fashion to that outlined within this protocol. Our protocol is an alternative to oral gavage of FITC-Dextran as outlined previously in Bio-protocol (Gupta et al., 2014).
- The skin glue used to seal our injection site on our tissue provides an adequate seal to prevent “leaking” of FITC-dextran into the peritoneal cavity of the mice. Care is taken to prevent leakage of the solution which might affect the overall results.
- Alternatives to suture closure of the abdomen include closure with clips/staples.
Acknowledgments
This project was supported in part by US National Institutes of Health (NIH) grants NIH-R01DK083325 (M.A.H), NIH P30 DK078392 (Digestive Health Center, Cincinnati Children’s Hospital Medical Center), and NIH UL1RR026314 (Clinical and Translational Science Awards (CTSA), University of Cincinnati).
References
- Dong, C. X., Zhao, W., Solomon, C., Rowland, K. J., Ackerley, C., Robine, S., Holzenberger, M., Gonska, T. and Brubaker, P. L. (2014). The intestinal epithelial insulin-like growth factor-1 receptor links glucagon-like peptide-2 action to gut barrier function. Endocrinology 155(2): 370-379.
- Gupta, J. and Nebreda, A. R. (2014). Analysis of intestinal permeability in mice. Bio-protocol 4 (22): e1289.
- Watson, C. L., Mahe, M. M., Munera, J., Howell, J. C., Sundaram, N., Poling, H. M., Schweitzer, J. I., Vallance, J. E., Mayhew, C. N., Sun, Y., Grabowski, G., Finkbeiner, S. R., Spence, J. R., Shroyer, N. F., Wells, J. M. and Helmrath, M. A. (2014). An in vivo model of human small intestine using pluripotent stem cells. Nat Med 20(11): 1310-1314.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Watson, C. L., Mahe, M. M. and Helmrath, M. A. (2015). In vivo Fluorescein Isothiocyanate-dextran (FD4) Permeability Assay. Bio-protocol 5(20): e1618. DOI: 10.21769/BioProtoc.1618.
Category
Cell Biology > Tissue analysis > Tissue staining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









