- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Generation of Mouse iNKT Cell Lines
Published: Vol 3, Iss 6, Mar 20, 2013 DOI: 10.21769/BioProtoc.419 Views: 15427

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2166 Views
Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1303 Views
Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1309 Views
Abstract
Natural killer T (NKT) cells bridge the innate and adaptive arms of the immune system, and manipulating their effector functions can have therapeutic significances in the treatment of autoimmunity, transplant biology, infectious disease and cancer. This important lymphocyte subset regulates the immune system through their potent cytokine production following the recognition of lipid antigen present in the context of the MHC class I-like CD1d molecule, in addition their ability to directly mediate cytotoxicity. Here, we describe a method of expanding mouse invariant NKT (iNKT) cell lines from mononuclear cells isolated from the thymus, spleen, or liver using bone marrow derived dendritic cells. These iNKT cell lines can be used study their co-signaling requirements, cytokine profiles and cytotoxic functions which will greatly enhance our knowledge of iNKT cell biology.
Materials and Reagents
- 6-12 weeks old C57BL/6 or BALB/c mice
- Erythrocyte lysis buffer- ACK Lysing buffer (Life Technologies, Gibco®, catalog number: A10492-01 )
- PBS-no calcium, no magnesium, 1x & 10x (Life Technologies, Gibco®, catalog number: 14190 )
- FITC-anti-CD3 (BD Biosciences, catalog number: clone 2C11; 553061 )
- APC-conjugated CD1d tetramer loaded with PBS-57 lipid antigen (National Institutes of Health Tetramer Core Facility, Atlanta, GA)
- PE-anti-NK1.1 (BD Biosciences, catalog number: clone PK136; 561046 )
- Recombinant mouse GM-CSF (R&D systems, catalog number: 415-GM )
- Recombinant mouse IL-4 (R&D systems, catalog number: 404-IL )
- Recombinant mouse IL-2 (Peprotech, catalog number: 212-12 )
- Recombinant mouse IL-7 (Peprotech, catalog number: 217-17 )
- Mouse Pan T cell isolation kit (MiltenyiBiotec, catalog number: 130-095-130 )
- Anti-APC microbeads (MiltenyiBiotec, catalog number: 130-090-855 )
- α-galactosylceramide (α-GalCer, KRN7000) (Enzo Life Sciences, catalog number: BML-SL232 )
- Lympholyte-M (Accurate Chemical)
- Percoll (Amersham-Pharmacia Biotech, catalog number: 17-0891-01 )
- RPMI-1640 medium (Life Technologies, Gibco®, catalog number: 11875 )
- Non-essential vitamin solution (Life Technologies, Gibco®, catalog number: 11140-050 )
- MEM Vitamin solution (Life Technologies, Gibco®, catalog number: 11120-052 )
- Sodium Pyruvate (Life Technologies, Gibco®, catalog number: 11360-070 )
- 2-mercaptoethanol (Life Technologies, Gibco®, catalog number: 21985-023 )
- Anti-CD16/32 antibody (Biolegend, catalog number: 101320 )
- Antibiotics: Penicillin-streptomycin
- Heat inactivated fetal bovine serum
- FBS
- EDTA
- Complete medium (see Recipes)
- MACS Buffer (see Recipes)
- Liver MNC isolation (see Recipes)
- Cell buffer solution (see Recipes)
Equipment
- 70 μm nylon mesh cell strainer (BD Bioscience, Falcon®, catalog number: 352350 )
- Centrifuge with swing out rotor and capable of 300-700 x g
- BD LSR II flow cytometer (BD Biosciences)
- Gamma irradiator
- 0.22 μm filter
Procedure
- To generate immature dendritic cells (BMDCs):
- Collect bone marrow from femurs of mice by passing through a 70 μm filter and centrifuge for 5 min at 300 x g. Discard supernatant and lyse red blood cells by gently resuspending the cell pellet in 5 ml ACK lysing buffer and incubating for 2-3 min at room temperature. Then quickly add 5 ml cell buffer solution and centrifuge for 5 min at 300 x g. Wash 2x with 10 ml cold cell buffer solution.
- Resuspend pelleted cells (106/ml) in complete medium containing 10 ng/ml mouse GM-CSF.
- Plate 5 x 106 cells/well in a 6 well plate for 7 days to generate immature DC.
- Collect bone marrow from femurs of mice by passing through a 70 μm filter and centrifuge for 5 min at 300 x g. Discard supernatant and lyse red blood cells by gently resuspending the cell pellet in 5 ml ACK lysing buffer and incubating for 2-3 min at room temperature. Then quickly add 5 ml cell buffer solution and centrifuge for 5 min at 300 x g. Wash 2x with 10 ml cold cell buffer solution.
- To isolate iNKT cells:
- Make a single-cell suspension of thymocytes or splenocytes by pressing organs through a 70 μm cell strainer (for liver see detailed protocol below) using cell buffer solution and the plunger from a 3 ml syringe.
Note: This protocol should be performed using 4-6 mice. The total number of NKT cells per organ is approximately one million, however typical yields are 30-40% per organ. - Centrifuge single cell suspension for 5 min at 300 x g. Discard supernatant and lyse red blood cells by gently resuspending the cell pellet in 10 ml ACK lysing buffer and incubating for 2-3 min at room temperature. Then quickly add 10 ml cell buffer solution and centrifuge for 5 min at 300 x g.
- Enrich T cells via negative selection by using the mouse Pan T Isolation Kit II according to the manufacturer’s protocol.
- Resuspend the cells (108/ml) in MACS buffer, and then select iNKT cells by incubating in the dark on ice with APC-conjugated CD1d tetramer loaded with PBS-57 lipid antigen (5-10 μl/ml of cells; 50 μg/ml) for 30 min.
- Next sort iNKT cells by using anti-APC beads following the manufacturer’s protocol.
- Make a single-cell suspension of thymocytes or splenocytes by pressing organs through a 70 μm cell strainer (for liver see detailed protocol below) using cell buffer solution and the plunger from a 3 ml syringe.
- For Liver Mononuclear cell (MNC) isolation:
Isolation of hepatic MNC (use 1 liver per tube):- Euthanize mice, open peritoneal cavity, slide intestines over to the right to expose the underside of the liver. Then using a 10 ml syringe and a 21 ga needle, inject 10 ml room tem PBS into the hepatic portal vein.
- Remove gall bladder, excise liver, place in tube containing cell buffer solution (on ice).
- Mince with liver tissue into very small pieces with scissors (500-700 cuts; < 3 mm3), and add to nylon mesh cell strainer on top of a 50 ml centrifuge tube. Add 2-3 ml cold cell buffer solution and mash through the mesh with the plunger from a 3 ml syringe. This does not need to be performed on ice, but tissue should be kept cold by the addition of ice-cold cell buffer solution and this step should be done quickly.
- Rinse with lots of cold cell buffer solution, continue mashing through and bring volume up to 40 ml with cold cell buffer solution. Centrifuge at 300 x g for 7 min at 4 °C.
- Resuspend pellet in 25 ml room temperature Percoll solution.
- Spin at 700 x g at room temperature for 12 min with the brake on (the cells of interest will form pellet so brake can be left on).
- Aspirate off supernatant (MNC are in the pellet), but be careful initially to remove the top layer of hepatocytes before aspirating down to the pellet. This will reduce the amount of hepatocyte contamination of MNC.
- Resuspend the pellet in 5 ml of ACK lysing buffer (or a similar RBC lysing buffer) to lyse the RBC, transfer to a 15 ml conical centrifuge tube, stop reaction by adding 5 ml of cell buffer solution. Spin at 300 x g for 7 min at 4 °C.
- Wash cell pellet 2x in 5-10 ml cell buffer solution. Resuspend in 5 ml cell buffer solution, media, or staining buffer and count (expect about 3-5 x 106 MNC)
- Continue as described above starting with step 3-e.
- Euthanize mice, open peritoneal cavity, slide intestines over to the right to expose the underside of the liver. Then using a 10 ml syringe and a 21 ga needle, inject 10 ml room tem PBS into the hepatic portal vein.
- In vitro expansion of iNKT cells:
- Collect the immature DCs by pipetting vigorously with ice cold complete medium and wash cells with 10 ml complete medium.
- Resuspend cells in 10 ml fresh medium and irradiate with 2,000 rads.
- Incubate 2 x 106 NKT cells with 2 x 105 irradiated immature DCs in the presence of α-GalCer (100 ng/ml) in 10 ml complete medium, and add 2 ml per well in 24 well plate.
- On day 4, add IL-2 (10 U/ml) and IL-7 (10 ng/ml) to the media.
- On day 10, harvest the cells and remove dead cells and debris using lympholyte-M according to the manufacturer’s protocol.
- Then re-stimulate the cells with immature DCs in the presence of 100 ng/ml α-GalCer and 10 U/ml mouse IL-2 at a 1:1 ratio as described in step c.
- Culture cells for 7-10 days, and replace medium every 4 days as step 4-d.
Note: If the cells are maintained in high levels of a-GalCer and cytokines, some specificity and sensitivity to CD1d is lost, so it is best to supplement with a-GalCer and cytokines when the cell line needs to be maintained, but add fresh complete media only if the cells are to be used in functional assays. - Flow cytometric analysis of expanding iNKT cells.
Gate on lymphocytes and check purity by flow cytometric analysis, using CD1d tetramer (or NK1.1 in C57BL/6 mice) and anti-CD3, see Figure 1.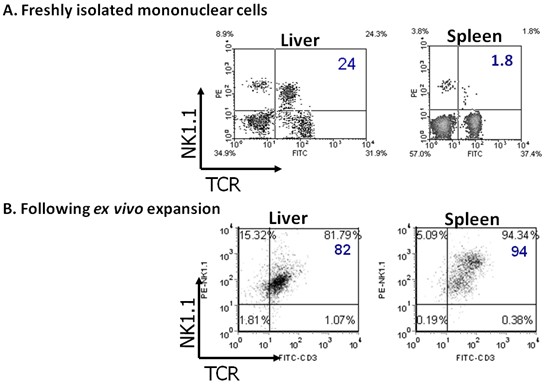
Figure 1.
- Collect 1 x 105 cells, and transfer into 1.5 ml tube, and filled with 1 ml FACS buffer (0.2% FBS in PBS).
- Centrifuge cells at 600 x g for 5 min, and then discard supernatant.
- Resuspend cells in 100 μl FACS buffer, and add 1 μl anti-CD16/32 antibody for 15 min to block non-specific binding, and then wash as step a.
- Resuspend cells in 100 μl FACS buffer, and add 0.5 μl APC-PBS57 loaded CD1d tetramer, or 1 μl PE-anti NK1.1 and 1 μl FITC anti-CD3 antibody (for 30 min on ice in dark, and then wash as step a.
- Resuspend cells in 200 μl PBS, and run samples on LSRII FACS machine.
- Collect the immature DCs by pipetting vigorously with ice cold complete medium and wash cells with 10 ml complete medium.
Recipes
- Complete medium
RPMI medium
100 mM sodium pyruvate
10 mM non-essential vitamin solution
100 mM MEM Vitamin solution
5 x 105 M 2-mercaptoethanol
50 U/ml penicillin-streptomycin
10% heat inactivated fetal bovine serum - MACS buffer
1 L PBS free of Ca2+ and Mg2+
5 g BSA
2 mmol EDTA
sterilized by passing through 0.22 μm filter - For Liver MNC isolation
Preparation of isotonic Percoll: Make up a 37.5% stock of PercoIl
337.5 ml of Percoll
100 ml of 10x PBS free of Ca2+ and Mg2+
562.5 ml ddH2O
Filter sterilize through a 0.2 micron filter unit
Store at 4 °C (very stable as long as it is kept sterile) - Cell buffer solution
Prepare 1x PBS supplemented with 2% FBS and 0.02% sodium azide
Acknowledgments
This work was supported by National Institutes of Health (NIH), National Cancer Institute Grants K01 CA131487, R21 CA162273, and R21 CA162277 and grants from the P30 Tumor Immunology and Immunotherapy Program to T.J.W., NIH AI 70258 to M. Tsuji, the NIH AI 44129, CA 108835, and P01 AI072677 to J.P. Schneck. The method was published in Webb et al. (2012) and it is an adaptation of the methods used by Tupin and Kronenberg (2006).
References
- Dellabona, P., Padovan, E., Casorati, G., Brockhaus, M. and Lanzavecchia, A. (1994). An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J Exp Med 180(3): 1171-1176.
- Exley, M., Garcia, J., Balk, S. P. and Porcelli, S. (1997). Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J Exp Med 186(1): 109-120.
- Fowlkes, B. J., Kruisbeek, A. M., Ton-That, H., Weston, M. A., Coligan, J. E., Schwartz, R. H. and Pardoll, D. M. (1987). A novel population of T-cell receptor alpha beta-bearing thymocytes which predominantly expresses a single V beta gene family. Nature 329(6136): 251-254.
- Harada, Y., Imataki, O., Heike, Y., Kawai, H., Shimosaka, A., Mori, S., Kami, M., Tanosaki, R., Ikarashi, Y., Iizuka, A., Yoshida, M., Wakasugi, H., Saito, S., Takaue, Y., Takei, M. and Kakizoe, T. (2005). Expansion of alpha-galactosylceramide-stimulated Valpha24+ NKT cells cultured in the absence of animal materials. J Immunother 28(4): 314-321.
- Prigozy, T. I., Naidenko, O., Qasba, P., Elewaut, D., Brossay, L., Khurana, A., Natori, T., Koezuka, Y., Kulkarni, A. and Kronenberg, M. (2001). Glycolipid antigen processing for presentation by CD1d molecules. Science 291(5504): 664-667.
- Shiratsuchi, T., Schneck, J., Kawamura, A. and Tsuji, M. (2009). Human CD1 dimeric proteins as indispensable tools for research on CD1-binding lipids and CD1-restricted T cells. J Immunol Methods 345(1-2): 49-59.
- Tupin, E. and Kronenberg, M. (2006). Activation of natural killer T cells by glycolipids. Methods Enzymol 417: 185-201.
- Webb, T. J., Li, X., Giuntoli, R. L., 2nd, Lopez, P. H., Heuser, C., Schnaar, R. L., Tsuji, M., Kurts, C., Oelke, M. and Schneck, J. P. (2012). Molecular identification of GD3 as a suppressor of the innate immune response in ovarian cancer. Cancer Res 72(15): 3744-3752.
- Webb, T. J., Bieler, J. G., Schneck, J. P. and Oelke, M. (2009). Ex vivo induction and expansion of natural killer T cells by CD1d1-Ig coated artificial antigen presenting cells. J Immunol Methods 346(1-2): 38-44.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Li, X., Tsuji, M., Schneck, J. and Webb, T. J. (2013). Generation of Mouse iNKT Cell Lines. Bio-protocol 3(6): e419. DOI: 10.21769/BioProtoc.419.
Category
Immunology > Immune cell isolation > Lymphocyte
Cell Biology > Cell isolation and culture > Cell isolation
Immunology > Immune cell isolation > Maintenance and differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












